(805) 483 1185

Home » About Us

About FOMAT Medical
FOMAT Medical Research, headquartered in Oxnard, California, is an Integrated Research Organization (IRO) focused on innovating healthcare with our high-quality services. With over 10 years of experience in participating in Phase 1 through Phase 4 clinical trials in a wide variety of therapeutic areas, we rely on a highly experienced clinical research team with the professional expertise necessary to assist Sponsors and Contract Research Organizations (CROs) with reaching their project goals quickly and effectively.
Our sites are pre-qualified to ensure their performance will exceed expectations as quickly and effectively as possible. As soon as they enter our network, our sites abide by internationally recognized Good Clinical Practices (GCP) to ensure clinical trials are conducted ethically and effectively. At FOMAT Medical Research, we facilitate every stage of the clinical trial process and provide high-quality services and solutions for sponsors, CROs, and sites.
Our Mission
Our mission is to diversify clinical trials and help bring innovations in healthcare to underrepresented populations ( while ensuring that Good Clinical Practice standards and ethics are upheld ) . Contributing high-quality data allows the scientific community to pursue and create better treatments for various health conditions.
Our Commitment
To Patients and Volunteers: We are committed to providing a safe and hospitable environment (while upholding good ethical standards) as we strive to improve healthcare treatment outcomes for you and future patients.
To S ponsors and CROs : We are committed to upholding excellence and high-quality management in the conduction of our clinical trials. One of our aims is to help you diversify clinical trial data.
Our Core Values
Keeps promises.
Own your commitments, hit timelines, reset expectations proactively.
Easy to Work With
Positive, collaborative, and respectful.

Willing to Learn
Open to feedback, desire to improve, able to adapt.
Winner’s mentality
Outcome-focused, solve problems, team first.
Our Leadership Team

Nicholas Focil
Chief executive officer.

Augusto Focil, M.D., M.P.H.
Chief medical officer.

Simon Corman
Chief growth officer.

Maria Fernanda Mancheno
Vp of quality and regulatory affairs.

Jonathan Gardow
Vp of business development.

Susana Moyano, M.D.
Director of clinical operations.

We Truly Believe In Diversity
To fully understand the full scope of new and existing treatments- every culture, nationality, and individual can provide crucial data and knowledge toward a better tomorrow.
We fully understand the importance of having diversity among study participants to accurately portray the general population. Therefore, we aim to uphold various principles that will help maintain patient diversity amongst our sites.
Explore our commitment to diversity. Ready to dive in?
Our staff's ethnicity.
No Data Found
Our Foreign Language Skills
If you would like to discuss how fomat can help you with your next clinical trial, allergy & immunology, dermatology, family medicine, gastroenterology, infectious disease, internal medicine & vaccines, obstetrics & gynecology, ophthalmology, rheumatology, top 2022 finalist of site patient recruitment innovation award.

Top 2024 Finalist

"Innovative Company of the Year" by the Oxnard Chamber of Commerce

Nicholas Focil is the founder and CEO of FOMAT Medical Research- an Integrated Research Organization with over 10 years of experience conducting clinical research in a wide variety of therapeutic areas. As CEO of a medical research company, Nicholas aims to diversify clinical trials, bring innovative healthcare to underrepresented populations, and ensure that high-quality data is contributed to the scientific community to create better treatments. Due to his efforts in empowering patients and diversifying clinical trials, University of California Riverside, his alma mater, featured Nicholas in their Spring 2022 inaugural list recognizing 40 remarkable alumni under the age of 40. Nicholas is currently chairman and co-founder of AlzWell, an app chosen by the Northern California MIT Healthcare Association as a top 100 healthcare startup (and is part of the Plug and Play ecosystem). Nicholas is an Executive Board Member of hyperCORE International, the second-largest research organization in the world. He has served as a member of both the Innovation Steering Committee of ACRP and Forbes Los Angeles Business Council. Over the past 15 years, Nicholas has contributed to numerous publications, held a variety of board member assignments, and been honored with many speaking engagements within the scientific and business fields.

Augusto Focil, MD, MPH
Dr. augusto focil, md, mph, is integral to the medical community (with close involvement in the ventura and oxnard community). with over 30 years dedicated to research, his expertise spans conditions such as osteoporosis, diabetes, nash, and obesity. notably, he played a pivotal role in covid-19 research during the pandemic. a proud alumnus of ucla school of medicine, he specialized in preventive medicine and further honed his skills with a master’s in family health & public health. dr. focil's commitment is not limited solely to research- he has been a leading recruiter in clinical trials and has worked closely within the oxnard community for over three decades. his unparalleled dedication stands as a testament to clinical excellence..

Simon Corman has been a leading figure in clinical research for over 20 years. His expertise in corporate and business development has helped form strategic partnerships with CROs and Sponsors and supported public-private clinical research collaborations. Adept in new tech implementation and diverse business models, Simon has spurred growth in innovative companies. He's shone in senior clinical research roles, spanning Central IRB to Full-Service CRO. He has also championed digital transformation in eConsent, CTMS, Decentralized Trials, and more. Simon notably led one of North America's premier IRBs, revolutionizing financial and clinical operations with cloud systems. His leadership through AAHRP accreditation and steering the launch of the industry's second-largest network of centers/IROs further underscores his immense clinical research expertise.

Fernanda Mancheno, is a distinguished professional with an impressive educational background. She earned her Master's in Accounting and Finance with a Specialization in Taxation from Universidad Particular de Especialidades Espíritu Santo (UEES). Additionally, she holds a degree in Foreign Trade Engineering with a focus on International Business from UTEG. Since 2013, Fernanda has been a pivotal member of FOMAT. Starting as a Project Manager in Regulatory Affairs, she swiftly rose to the position of Regional Clin-Ops and Regulatory Director for Latin America. Today, she proudly serves as the Vice President of Quality & Regulatory Affairs. Her commitment is evident in her leadership across multiple clinical trials and phases. Collaborating with entities like the local Ministries of Health in Ecuador and Colombia, Ethics Committees, and IRBs- she's been instrumental in securing over 500 regulatory approvals for clinical trials. Fernanda's achievements solidify her prominence in her field.

Jon Gardow has consulted with the drug development industry for over 13 years, working with sponsors, CROs, research sites, and clinical technology. His understanding of the interconnected markets has fostered successful collaborations between buyers and sellers across the pharmaceutical space and helped build organizations at the executive level. Jon firmly believes in making clinical trials more accessible to the world's population and has evangelized on behalf of the industry's leading clinical payments provider to modernize how sites and participants receive compensation. In 2017, Jon co-founded a nationwide clinical staffing organization and served as its President and Head of Business Development to partner with growing organizations and help them expand their presence into new therapeutic indications and product lines. Jon now serves FOMAT as the Vice President of Business Development, where his efforts seek to amplify the organization's successful track record of performing high-quality clinical research services and partner with innovative drug development experts to bring new treatments to market.
- PRO Courses Guides New Tech Help Pro Expert Videos About wikiHow Pro Upgrade Sign In
- EDIT Edit this Article
- EXPLORE Tech Help Pro About Us Random Article Quizzes Request a New Article Community Dashboard This Or That Game Forums Popular Categories Arts and Entertainment Artwork Books Movies Computers and Electronics Computers Phone Skills Technology Hacks Health Men's Health Mental Health Women's Health Relationships Dating Love Relationship Issues Hobbies and Crafts Crafts Drawing Games Education & Communication Communication Skills Personal Development Studying Personal Care and Style Fashion Hair Care Personal Hygiene Youth Personal Care School Stuff Dating All Categories Arts and Entertainment Finance and Business Home and Garden Relationship Quizzes Cars & Other Vehicles Food and Entertaining Personal Care and Style Sports and Fitness Computers and Electronics Health Pets and Animals Travel Education & Communication Hobbies and Crafts Philosophy and Religion Work World Family Life Holidays and Traditions Relationships Youth
- Browse Articles
- Learn Something New
- Quizzes Hot
- Happiness Hub
- This Or That Game
- Train Your Brain
- Explore More
- Support wikiHow
- About wikiHow
- Log in / Sign up
- Education and Communications
- College University and Postgraduate
- Academic Writing
- Research Papers
How to Write a Medical Research Paper
Last Updated: August 12, 2024 Approved
This article was co-authored by Chris M. Matsko, MD . Dr. Chris M. Matsko is a retired physician based in Pittsburgh, Pennsylvania. With over 25 years of medical research experience, Dr. Matsko was awarded the Pittsburgh Cornell University Leadership Award for Excellence. He holds a BS in Nutritional Science from Cornell University and an MD from the Temple University School of Medicine in 2007. Dr. Matsko earned a Research Writing Certification from the American Medical Writers Association (AMWA) in 2016 and a Medical Writing & Editing Certification from the University of Chicago in 2017. wikiHow marks an article as reader-approved once it receives enough positive feedback. In this case, 89% of readers who voted found the article helpful, earning it our reader-approved status. This article has been viewed 207,078 times.
Writing a medical research paper is similar to writing other research papers in that you want to use reliable sources, write in a clear and organized style, and offer a strong argument for all conclusions you present. In some cases the research you discuss will be data you have actually collected to answer your research questions. Understanding proper formatting, citations, and style will help you write and informative and respected paper.
Researching Your Paper

- Pick something that really interests you to make the research more fun.
- Choose a topic that has unanswered questions and propose solutions.

- Quantitative studies consist of original research performed by the writer. These research papers will need to include sections like Hypothesis (or Research Question), Previous Findings, Method, Limitations, Results, Discussion, and Application.
- Synthesis papers review the research already published and analyze it. They find weaknesses and strengths in the research, apply it to a specific situation, and then indicate a direction for future research.

- Keep track of your sources. Write down all publication information necessary for citation: author, title of article, title of book or journal, publisher, edition, date published, volume number, issue number, page number, and anything else pertaining to your source. A program like Endnote can help you keep track of your sources.
- Take detailed notes as you read. Paraphrase information in your own words or if you copy directly from the article or book, indicate that these are direct quotes by using quotation marks to prevent plagiarism.
- Be sure to keep all of your notes with the correct source.
- Your professor and librarians can also help you find good resources.

- Keep all of your notes in a physical folder or in a digitized form on the computer.
- Start to form the basic outline of your paper using the notes you have collected.
Writing Your Paper

- Start with bullet points and then add in notes you've taken from references that support your ideas. [1] X Trustworthy Source PubMed Central Journal archive from the U.S. National Institutes of Health Go to source
- A common way to format research papers is to follow the IMRAD format. This dictates the structure of your paper in the following order: I ntroduction, M ethods, R esults, a nd D iscussion. [2] X Research source
- The outline is just the basic structure of your paper. Don't worry if you have to rearrange a few times to get it right.
- Ask others to look over your outline and get feedback on the organization.
- Know the audience you are writing for and adjust your style accordingly. [3] X Research source

- Use a standard font type and size, such as Times New Roman 12 point font.
- Double-space your paper.
- If necessary, create a cover page. Most schools require a cover page of some sort. Include your main title, running title (often a shortened version of your main title), author's name, course name, and semester.

- Break up information into sections and subsections and address one main point per section.
- Include any figures or data tables that support your main ideas.
- For a quantitative study, state the methods used to obtain results.

- Clearly state and summarize the main points of your research paper.
- Discuss how this research contributes to the field and why it is important. [4] X Research source
- Highlight potential applications of the theory if appropriate.
- Propose future directions that build upon the research you have presented. [5] X Research source
- Keep the introduction and discussion short, and spend more time explaining the methods and results.

- State why the problem is important to address.
- Discuss what is currently known and what is lacking in the field.
- State the objective of your paper.
- Keep the introduction short.

- Highlight the purpose of the paper and the main conclusions.
- State why your conclusions are important.
- Be concise in your summary of the paper.
- Show that you have a solid study design and a high-quality data set.
- Abstracts are usually one paragraph and between 250 – 500 words.

- Unless otherwise directed, use the American Medical Association (AMA) style guide to properly format citations.
- Add citations at end of a sentence to indicate that you are using someone else's idea. Use these throughout your research paper as needed. They include the author's last name, year of publication, and page number.
- Compile your reference list and add it to the end of your paper.
- Use a citation program if you have access to one to simplify the process.

- Continually revise your paper to make sure it is structured in a logical way.
- Proofread your paper for spelling and grammatical errors.
- Make sure you are following the proper formatting guidelines provided for the paper.
- Have others read your paper to proofread and check for clarity. Revise as needed.
Expert Q&A

- Ask your professor for help if you are stuck or confused about any part of your research paper. They are familiar with the style and structure of papers and can provide you with more resources. Thanks Helpful 0 Not Helpful 0
- Refer to your professor's specific guidelines. Some instructors modify parts of a research paper to better fit their assignment. Others may request supplementary details, such as a synopsis for your research project . Thanks Helpful 0 Not Helpful 0
- Set aside blocks of time specifically for writing each day. Thanks Helpful 0 Not Helpful 0

- Do not plagiarize. Plagiarism is using someone else's work, words, or ideas and presenting them as your own. It is important to cite all sources in your research paper, both through internal citations and on your reference page. Thanks Helpful 4 Not Helpful 2
You Might Also Like

- ↑ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3178846/
- ↑ http://owl.excelsior.edu/research-and-citations/outlining/outlining-imrad/
- ↑ http://china.elsevier.com/ElsevierDNN/Portals/7/How%20to%20write%20a%20world-class%20paper.pdf
- ↑ http://intqhc.oxfordjournals.org/content/16/3/191
- ↑ http://www.ruf.rice.edu/~bioslabs/tools/report/reportform.html#form
About This Article

To write a medical research paper, research your topic thoroughly and compile your data. Next, organize your notes and create a strong outline that breaks up the information into sections and subsections, addressing one main point per section. Write the results and discussion sections first to go over your findings, then write the introduction to state your objective and provide background information. Finally, write the abstract, which concisely summarizes the article by highlighting the main points. For tips on formatting and using citations, read on! Did this summary help you? Yes No
- Send fan mail to authors
Reader Success Stories
Joshua Benibo
Jun 5, 2018

Did this article help you?

Dominic Cipriano
Aug 16, 2016
Obiajulu Echedom
Apr 2, 2017
Noura Ammar Alhossiny
Feb 14, 2017
Dawn Daniel
Apr 20, 2017

Featured Articles

Trending Articles

Watch Articles

- Terms of Use
- Privacy Policy
- Do Not Sell or Share My Info
- Not Selling Info
wikiHow Tech Help Pro:
Develop the tech skills you need for work and life
Preparing a Manuscript for Submission to a Medical Journal
Page Contents
- General Principles
- Reporting Guidelines
- Introduction
- Illustrations (Figures)
- Units of Measurement
- Abbreviations and Symbols
1. General Principles
The text of articles reporting original research is usually divided into Introduction, Methods, Results, and Discussion sections. This so-called “IMRAD” structure is not an arbitrary publication format but a reflection of the process of scientific discovery. Articles often need subheadings within these sections to further organize their content. Other types of articles, such as meta-analyses, may require different formats, while case reports, narrative reviews, and editorials may have less structured or unstructured formats.
Electronic formats have created opportunities for adding details or sections, layering information, cross-linking, or extracting portions of articles in electronic versions. Supplementary electronic-only material should be submitted and sent for peer review simultaneously with the primary manuscript.
2. Reporting Guidelines
Reporting guidelines have been developed for different study designs; examples include CONSORT for randomized trials, STROBE for observational studies, PRISMA for systematic reviews and meta-analyses, and STARD for studies of diagnostic accuracy. Journals are encouraged to ask authors to follow these guidelines because they help authors describe the study in enough detail for it to be evaluated by editors, reviewers, readers, and other researchers evaluating the medical literature. Authors are encouraged to refer to the SAGER guidelines for reporting of sex and gender information in study design, data analyses, results, and interpretation of findings. Authors of review manuscripts are encouraged to describe the methods used for locating, selecting, extracting, and synthesizing data; this is mandatory for systematic reviews. Good sources for reporting guidelines are the EQUATOR Network and the NLM's Research Reporting Guidelines and Initiatives .
3. Manuscript Sections
The following are general requirements for reporting within sections of all study designs and manuscript formats.
a. Title Page
General information about an article and its authors is presented on a manuscript title page and usually includes the article title, author information, any disclaimers, sources of support, word count, and sometimes the number of tables and figures.
b. Abstract
Original research, systematic reviews, and meta-analyses require structured abstracts. The abstract should provide the context or background for the study and should state the study's purpose, basic procedures (selection of study participants, settings, measurements, analytical methods), main findings (giving specific effect sizes and their statistical and clinical significance, if possible), and principal conclusions. It should emphasize new and important aspects of the study or observations, note important limitations, and not overinterpret findings. Clinical trial abstracts should include items that the CONSORT group has identified as essential . Funding sources should be listed separately after the abstract to facilitate proper display and indexing for search retrieval by MEDLINE. The funding statement should include only direct support of the work described. General institutional support for an author's time on the work should be distinguished from direct overall funding of the work. An appropriate funding statement might be: “This study was funded by ABC; Dr. F's time on the work was supported by XYZ.”
Because abstracts are the only substantive portion of the article indexed in many electronic databases, and the only portion many readers read, authors need to ensure that they accurately reflect the content of the article. Unfortunately, information in abstracts often differs from that in the text. Authors and editors should work in the process of revision and review to ensure that information is consistent in both places. The format required for structured abstracts differs from journal to journal, and some journals use more than one format; authors need to prepare their abstracts in the format specified by the journal they have chosen.
The ICMJE recommends that journals publish the clinical trial registration number at the end of the abstract. The ICMJE also recommends that, when a registration number is available, authors list that number the first time they use a trial acronym to refer to the trial they are reporting or to other trials that they mention in the manuscript. If the data have been deposited in a public repository and/or are being used in a secondary analysis, authors should state at the end of the abstract the unique, persistent data set identifier, repository name and number.
c. Introduction
Provide a context or background for the study (that is, the nature of the problem and its significance). State the specific purpose or research objective of, or hypothesis tested by, the study or observation. Cite only directly pertinent references, and do not include data or conclusions from the work being reported.
The guiding principle of the Methods section should be clarity about how and why a study was done in a particular way. The Methods section should aim to be sufficiently detailed such that others with access to the data would be able to reproduce the results. In general, the section should include only information that was available at the time the plan or protocol for the study was being written; all information obtained during the study belongs in the Results section. If an organization was paid or otherwise contracted to help conduct the research (examples include data collection and management), then this should be detailed in the methods.
The Methods section should include a statement indicating that the research was approved by an independent local, regional or national review body (e.g., ethics committee, institutional review board). If doubt exists whether the research was conducted in accordance with the Helsinki Declaration, the authors must explain the rationale for their approach and demonstrate that the local, regional or national review body explicitly approved the doubtful aspects of the study. (See section II.E.)
Authors who used AI technology to conduct the study should describe its use in the methods section in sufficient detail to enable replication of the approach. including the tool used, version, and prompts where applicable.
i. Selection and Description of Participants
Clearly describe the selection of observational or experimental participants (healthy individuals or patients, including controls), including eligibility and exclusion criteria and a description of the source population. Because the relevance of such variables as age, sex, or ethnicity is not always known at the time of study design, researchers should aim for inclusion of representative populations into all study types and at a minimum provide descriptive data for these and other relevant demographic variables. Comment on how representative the study sample is of the larger population of interest.
Ensure correct use of the terms sex (when reporting biological factors) and gender (identity, psychosocial or cultural factors), and, unless inappropriate, report the sex and/or gender of study participants, the sex of animals or cells, and describe the methods used to determine sex and gender. If the study was done involving an exclusive population, for example in only one sex, authors should justify why. Authors should define how they determined race or ethnicity and justify their relevance. In the case where race or ethnicity was not collected, explain why it was not collected. Race and ethnicity are social and not biological constructs; authors should interpret results associated with race and ethnicity in that context. Authors should use neutral, precise, and respectful language to describe study participants and avoid the use of terminology that might stigmatize participants.
ii. Data Collection and Measurements
Specify the study's main and secondary objectives–usually identified as primary and secondary outcomes. Identify methods, equipment (give the manufacturer's name and address in parentheses), and procedures in sufficient detail to allow others to reproduce the results. Give references to established methods, including statistical methods (see below); provide references and brief descriptions for methods that have been published but are not well-known; describe new or substantially modified methods, give the reasons for using them, and evaluate their limitations. Identify precisely all drugs and chemicals used, including generic name(s), dose(s), and route(s) of administration. Identify appropriate scientific names and gene names.
iii. Statistics
Describe statistical methods with enough detail to enable a knowledgeable reader with access to the original data to judge its appropriateness for the study and to verify the reported results. When possible, quantify findings and present them with appropriate indicators of measurement error or uncertainty (such as confidence intervals). Avoid relying solely on statistical hypothesis testing, such as P values, which fail to convey important information about effect size and precision of estimates. References for the design of the study and statistical methods should be to standard works when possible (with pages stated). Define statistical terms, abbreviations, and most symbols. Specify the statistical software package(s) and versions used. Distinguish prespecified from exploratory analyses, including subgroup analyses.
Present your results in logical sequence in the text, tables, and figures, giving the main or most important findings first. Do not repeat all the data in the tables or figures in the text; emphasize or summarize only the most important observations. Provide data on all primary and secondary outcomes identified in the Methods Section. Extra or supplementary materials and technical details can be placed in an appendix where they will be accessible but will not interrupt the flow of the text, or they can be published solely in the electronic version of the journal.
Give numeric results not only as derivatives (for example, percentages) but also as the absolute numbers from which the derivatives were calculated. Restrict tables and figures to those needed to explain the argument of the paper and to assess supporting data. Use graphs as an alternative to tables with many entries; do not duplicate data in graphs and tables. Avoid nontechnical uses of technical terms in statistics, such as “random” (which implies a randomizing device), “normal,” “significant,” “correlations,” and “sample.”
Separate reporting of data by demographic variables, such as age and sex, facilitate pooling of data for subgroups across studies and should be routine, unless there are compelling reasons not to stratify reporting, which should be explained.
f. Discussion
It is useful to begin the discussion by briefly summarizing the main findings, and explore possible mechanisms or explanations for these findings. Emphasize the new and important aspects of your study and put your findings in the context of the totality of the relevant evidence. State the limitations of your study, and explore the implications of your findings for future research and for clinical practice or policy. Discuss the influence or association of variables, such as sex and/or gender, on your findings, where appropriate, and the limitations of the data. Do not repeat in detail data or other information given in other parts of the manuscript, such as in the Introduction or the Results section.
Link the conclusions with the goals of the study but avoid unqualified statements and conclusions not adequately supported by the data. In particular, distinguish between clinical and statistical significance, and avoid making statements on economic benefits and costs unless the manuscript includes the appropriate economic data and analyses. Avoid claiming priority or alluding to work that has not been completed. State new hypotheses when warranted, but label them clearly.
g. References
I. general considerations.
Authors should provide direct references to original research sources whenever possible. References should be made to published articles rather than to abstracts whenever possible. References should not be used by authors, editors, or peer reviewers to promote self-interests. Authors should avoid citing articles in predatory or pseudo-journals. When preprints are cited, the citation should clearly indicate that the reference is a preprint (also see Section III.D.3). Although references to review articles can be an efficient way to guide readers to a body of literature, review articles do not always reflect original work accurately. On the other hand, extensive lists of references to original work on a topic can use excessive space. Fewer references to key original papers often serve as well as more exhaustive lists, particularly since references can now be added to the electronic version of published papers, and since electronic literature searching allows readers to retrieve published literature efficiently.
References to papers accepted but not yet published should be designated as “in press” or “forthcoming.” Information from manuscripts submitted but not accepted should be cited in the text as “unpublished observations” with written permission from the source.
Published articles should reference the unique, persistent identifiers of the data sets employed.
Avoid citing a “personal communication” unless it provides essential information not available from a public source, in which case the name of the person and date of communication should be cited in parentheses in the text. For scientific articles, obtain written permission and confirmation of accuracy from the source of a personal communication.
Referencing AI-generated material as the primary source is not acceptable.
Some but not all journals check the accuracy of all reference citations; thus, citation errors sometimes appear in the published version of articles. To minimize such errors, references should be verified using either an electronic bibliographic source, such as PubMed, or print copies from original sources. Authors are responsible for checking that none of the references cite retracted articles except in the context of referring to the retraction. For articles published in journals indexed in MEDLINE, the ICMJE considers PubMed the authoritative source for information about retractions. Authors can identify retracted articles in MEDLINE by searching PubMed for "Retracted publication [pt]", where the term "pt" in square brackets stands for publication type, or by going directly to the PubMed's list of retracted publications .
References should be numbered consecutively in the order in which they are first mentioned in the text. Identify references in text, tables, and legends by Arabic numerals in parentheses.
References cited only in tables or figure legends should be numbered in accordance with the sequence established by the first identification in the text of the particular table or figure. The titles of journals should be abbreviated according to the style used for MEDLINE ( www.ncbi.nlm.nih.gov/nlmcatalog/journals ). Journals vary on whether they ask authors to cite electronic references within parentheses in the text or in numbered references following the text. Authors should consult with the journal to which they plan to submit their work.
ii. Style and Format
References should follow the standards summarized in the NLM’s Sample References webpage and detailed in the NLM’s Citing Medicine, 2nd edition . These resources are regularly updated as new media develop, and currently include guidance for print documents; unpublished material; audio and visual media; material on CD-ROM, DVD, or disk; and material on the Internet.
Tables capture information concisely and display it efficiently; they also provide information at any desired level of detail and precision. Including data in tables rather than text frequently makes it possible to reduce the length of the text.
Prepare tables according to the specific journal's requirements; to avoid errors it is best if tables can be directly imported into the journal's publication software. Number tables consecutively in the order of their first citation in the text and supply a title for each. Titles in tables should be short but self-explanatory, containing information that allows readers to understand the table's content without having to go back to the text. Be sure that each table is cited in the text.
Give each column a short or an abbreviated heading. Authors should place explanatory matter in footnotes, not in the heading. Explain all nonstandard abbreviations in footnotes, and use symbols to explain information if needed. Symbols may vary from journal to journal (alphabet letter or such symbols as *, †, ‡, §), so check each journal's instructions for authors for required practice. Identify statistical measures of variations, such as standard deviation and standard error of the mean.
If you use data from another published or unpublished source, obtain permission and acknowledge that source fully.
Additional tables containing backup data too extensive to publish in print may be appropriate for publication in the electronic version of the journal, deposited with an archival service, or made available to readers directly by the authors. An appropriate statement should be added to the text to inform readers that this additional information is available and where it is located. Submit such tables for consideration with the paper so that they will be available to the peer reviewers.
i. Illustrations (Figures)
Digital images of manuscript illustrations should be submitted in a suitable format for print publication. Most submission systems have detailed instructions on the quality of images and check them after manuscript upload. For print submissions, figures should be either professionally drawn and photographed, or submitted as photographic-quality digital prints.
For radiological and other clinical and diagnostic images, as well as pictures of pathology specimens or photomicrographs, send high-resolution photographic image files. Before-and-after images should be taken with the same intensity, direction, and color of light. Since blots are used as primary evidence in many scientific articles, editors may require deposition of the original photographs of blots on the journal's website.
Although some journals redraw figures, many do not. Letters, numbers, and symbols on figures should therefore be clear and consistent throughout, and large enough to remain legible when the figure is reduced for publication. Figures should be made as self-explanatory as possible, since many will be used directly in slide presentations. Titles and detailed explanations belong in the legends—not on the illustrations themselves.
Photomicrographs should have internal scale markers. Symbols, arrows, or letters used in photomicrographs should contrast with the background. Explain the internal scale and identify the method of staining in photomicrographs.
Figures should be numbered consecutively according to the order in which they have been cited in the text. If a figure has been published previously, acknowledge the original source and submit written permission from the copyright holder to reproduce it. Permission is required irrespective of authorship or publisher except for documents in the public domain.
In the manuscript, legends for illustrations should be on a separate page, with Arabic numerals corresponding to the illustrations. When symbols, arrows, numbers, or letters are used to identify parts of the illustrations, identify and explain each one clearly in the legend.
j. Units of Measurement
Measurements of length, height, weight, and volume should be reported in metric units (meter, kilogram, or liter) or their decimal multiples.
Temperatures should be in degrees Celsius. Blood pressures should be in millimeters of mercury, unless other units are specifically required by the journal.
Journals vary in the units they use for reporting hematologic, clinical chemistry, and other measurements. Authors must consult the Information for Authors of the particular journal and should report laboratory information in both local and International System of Units (SI).
Editors may request that authors add alternative or non-SI units, since SI units are not universally used. Drug concentrations may be reported in either SI or mass units, but the alternative should be provided in parentheses where appropriate.
k. Abbreviations and Symbols
Use only standard abbreviations; use of nonstandard abbreviations can be confusing to readers. Avoid abbreviations in the title of the manuscript. The spelled-out abbreviation followed by the abbreviation in parentheses should be used on first mention unless the abbreviation is a standard unit of measurement.
Next: Sending the Manuscript to the Journal
FAQ How do I format a specific citation?
View Answer
Keep up-to-date Request to receive an E-mail when the Recommendations are updated.
Subscribe to Changes
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Types of Study in Medical Research
Part 3 of a Series on Evaluation of Scientific Publications
Bernd Röhrig , Dr. rer. nat.
Jean-baptist du prel , dr. med., daniel wachtlin, maria blettner , prof. dr. rer. nat..
- Author information
- Article notes
- Copyright and License information
*MDK Rheinland-Pfalz, Referat Rehabilitation/Biometrie, Albiger Str. 19 d, 55232 Alzey, Germany, [email protected]
Received 2008 Jun 30; Accepted 2008 Nov 13; Issue date 2009 Apr.
The choice of study type is an important aspect of the design of medical studies. The study design and consequent study type are major determinants of a study’s scientific quality and clinical value.
This article describes the structured classification of studies into two types, primary and secondary, as well as a further subclassification of studies of primary type. This is done on the basis of a selective literature search concerning study types in medical research, in addition to the authors’ own experience.
Three main areas of medical research can be distinguished by study type: basic (experimental), clinical, and epidemiological research. Furthermore, clinical and epidemiological studies can be further subclassified as either interventional or noninterventional.
Conclusions
The study type that can best answer the particular research question at hand must be determined not only on a purely scientific basis, but also in view of the available financial resources, staffing, and practical feasibility (organization, medical prerequisites, number of patients, etc.).
Keywords: study type, basic research, clinical research, epidemiology, literature search
The quality, reliability and possibility of publishing a study are decisively influenced by the selection of a proper study design. The study type is a component of the study design (see the article "Study Design in Medical Research") and must be specified before the study starts. The study type is determined by the question to be answered and decides how useful a scientific study is and how well it can be interpreted. If the wrong study type has been selected, this cannot be rectified once the study has started.
After an earlier publication dealing with aspects of study design, the present article deals with study types in primary and secondary research. The article focuses on study types in primary research. A special article will be devoted to study types in secondary research, such as meta-analyses and reviews. This article covers the classification of individual study types. The conception, implementation, advantages, disadvantages and possibilities of using the different study types are illustrated by examples. The article is based on a selective literature research on study types in medical research, as well as the authors’ own experience.
Classification of study types
In principle, medical research is classified into primary and secondary research. While secondary research summarizes available studies in the form of reviews and meta-analyses, the actual studies are performed in primary research. Three main areas are distinguished: basic medical research, clinical research, and epidemiological research. In individual cases, it may be difficult to classify individual studies to one of these three main categories or to the subcategories. In the interests of clarity and to avoid excessive length, the authors will dispense with discussing special areas of research, such as health services research, quality assurance, or clinical epidemiology. Figure 1 gives an overview of the different study types in medical research.
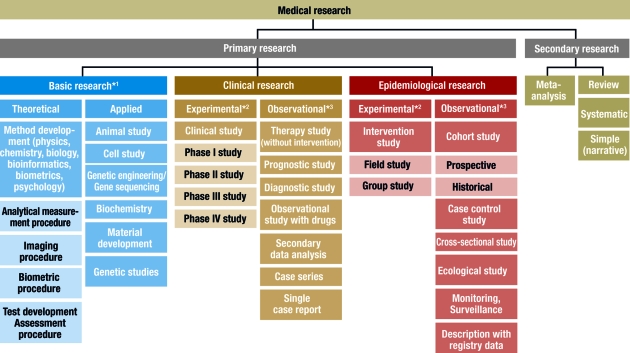
Classification of different study types
*1 , sometimes known as experimental research; *2 , analogous term: interventional; *3 , analogous term: noninterventional or nonexperimental
This scheme is intended to classify the study types as clearly as possible. In the interests of clarity, we have excluded clinical epidemiology — a subject which borders on both clinical and epidemiological research ( 3 ). The study types in this area can be found under clinical research and epidemiology.
Basic research
Basic medical research (otherwise known as experimental research) includes animal experiments, cell studies, biochemical, genetic and physiological investigations, and studies on the properties of drugs and materials. In almost all experiments, at least one independent variable is varied and the effects on the dependent variable are investigated. The procedure and the experimental design can be precisely specified and implemented ( 1 ). For example, the population, number of groups, case numbers, treatments and dosages can be exactly specified. It is also important that confounding factors should be specifically controlled or reduced. In experiments, specific hypotheses are investigated and causal statements are made. High internal validity (= unambiguity) is achieved by setting up standardized experimental conditions, with low variability in the units of observation (for example, cells, animals or materials). External validity is a more difficult issue. Laboratory conditions cannot always be directly transferred to normal clinical practice and processes in isolated cells or in animals are not equivalent to those in man (= generalizability) ( 2 ).
Basic research also includes the development and improvement of analytical procedures—such as analytical determination of enzymes, markers or genes—, imaging procedures—such as computed tomography or magnetic resonance imaging—, and gene sequencing—such as the link between eye color and specific gene sequences. The development of biometric procedures—such as statistical test procedures, modeling and statistical evaluation strategies—also belongs here.
Clinical studies
Clinical studies include both interventional (or experimental) studies and noninterventional (or observational) studies. A clinical drug study is an interventional clinical study, defined according to §4 Paragraph 23 of the Medicines Act [Arzneimittelgesetz; AMG] as "any study performed on man with the purpose of studying or demonstrating the clinical or pharmacological effects of drugs, to establish side effects, or to investigate absorption, distribution, metabolism or elimination, with the aim of providing clear evidence of the efficacy or safety of the drug."
Interventional studies also include studies on medical devices and studies in which surgical, physical or psychotherapeutic procedures are examined. In contrast to clinical studies, §4 Paragraph 23 of the AMG describes noninterventional studies as follows: "A noninterventional study is a study in the context of which knowledge from the treatment of persons with drugs in accordance with the instructions for use specified in their registration is analyzed using epidemiological methods. The diagnosis, treatment and monitoring are not performed according to a previously specified study protocol, but exclusively according to medical practice."
The aim of an interventional clinical study is to compare treatment procedures within a patient population, which should exhibit as few as possible internal differences, apart from the treatment ( 4 , e1 ). This is to be achieved by appropriate measures, particularly by random allocation of the patients to the groups, thus avoiding bias in the result. Possible therapies include a drug, an operation, the therapeutic use of a medical device such as a stent, or physiotherapy, acupuncture, psychosocial intervention, rehabilitation measures, training or diet. Vaccine studies also count as interventional studies in Germany and are performed as clinical studies according to the AMG.
Interventional clinical studies are subject to a variety of legal and ethical requirements, including the Medicines Act and the Law on Medical Devices. Studies with medical devices must be registered by the responsible authorities, who must also approve studies with drugs. Drug studies also require a favorable ruling from the responsible ethics committee. A study must be performed in accordance with the binding rules of Good Clinical Practice (GCP) ( 5 , e2 – e4 ). For clinical studies on persons capable of giving consent, it is absolutely essential that the patient should sign a declaration of consent (informed consent) ( e2 ). A control group is included in most clinical studies. This group receives another treatment regimen and/or placebo—a therapy without substantial efficacy. The selection of the control group must not only be ethically defensible, but also be suitable for answering the most important questions in the study ( e5 ).
Clinical studies should ideally include randomization, in which the patients are allocated by chance to the therapy arms. This procedure is performed with random numbers or computer algorithms ( 6 – 8 ). Randomization ensures that the patients will be allocated to the different groups in a balanced manner and that possible confounding factors—such as risk factors, comorbidities and genetic variabilities—will be distributed by chance between the groups (structural equivalence) ( 9 , 10 ). Randomization is intended to maximize homogeneity between the groups and prevent, for example, a specific therapy being reserved for patients with a particularly favorable prognosis (such as young patients in good physical condition) ( 11 ).
Blinding is another suitable method to avoid bias. A distinction is made between single and double blinding. With single blinding, the patient is unaware which treatment he is receiving, while, with double blinding, neither the patient nor the investigator knows which treatment is planned. Blinding the patient and investigator excludes possible subjective (even subconscious) influences on the evaluation of a specific therapy (e.g. drug administration versus placebo). Thus, double blinding ensures that the patient or therapy groups are both handled and observed in the same manner. The highest possible degree of blinding should always be selected. The study statistician should also remain blinded until the details of the evaluation have finally been specified.
A well designed clinical study must also include case number planning. This ensures that the assumed therapeutic effect can be recognized as such, with a previously specified statistical probability (statistical power) ( 4 , 6 , 12 ).
It is important for the performance of a clinical trial that it should be carefully planned and that the exact clinical details and methods should be specified in the study protocol ( 13 ). It is, however, also important that the implementation of the study according to the protocol, as well as data collection, must be monitored. For a first class study, data quality must be ensured by double data entry, programming plausibility tests, and evaluation by a biometrician. International recommendations for the reporting of randomized clinical studies can be found in the CONSORT statement (Consolidated Standards of Reporting Trials, www.consort-statement.org ) ( 14 ). Many journals make this an essential condition for publication.
For all the methodological reasons mentioned above and for ethical reasons, the randomized controlled and blinded clinical trial with case number planning is accepted as the gold standard for testing the efficacy and safety of therapies or drugs ( 4 , e1 , 15 ).
In contrast, noninterventional clinical studies (NIS) are patient-related observational studies, in which patients are given an individually specified therapy. The responsible physician specifies the therapy on the basis of the medical diagnosis and the patient’s wishes. NIS include noninterventional therapeutic studies, prognostic studies, observational drug studies, secondary data analyses, case series and single case analyses ( 13 , 16 ). Similarly to clinical studies, noninterventional therapy studies include comparison between therapies; however, the treatment is exclusively according to the physician’s discretion. The evaluation is often retrospective. Prognostic studies examine the influence of prognostic factors (such as tumor stage, functional state, or body mass index) on the further course of a disease. Diagnostic studies are another class of observational studies, in which either the quality of a diagnostic method is compared to an established method (ideally a gold standard), or an investigator is compared with one or several other investigators (inter-rater comparison) or with himself at different time points (intra-rater comparison) ( e1 ). If an event is very rare (such as a rare disease or an individual course of treatment), a single-case study, or a case series, are possibilities. A case series is a study on a larger patient group with a specific disease. For example, after the discovery of the AIDS virus, the Center for Disease Control (CDC) in the USA collected a case series of 1000 patients, in order to study frequent complications of this infection. The lack of a control group is a disadvantage of case series. For this reason, case series are primarily used for descriptive purposes ( 3 ).
Epidemiological studies
The main point of interest in epidemiological studies is to investigate the distribution and historical changes in the frequency of diseases and the causes for these. Analogously to clinical studies, a distinction is made between experimental and observational epidemiological studies ( 16 , 17 ).
Interventional studies are experimental in character and are further subdivided into field studies (sample from an area, such as a large region or a country) and group studies (sample from a specific group, such as a specific social or ethnic group). One example was the investigation of the iodine supplementation of cooking salt to prevent cretinism in a region with iodine deficiency. On the other hand, many interventions are unsuitable for randomized intervention studies, for ethical, social or political reasons, as the exposure may be harmful to the subjects ( 17 ).
Observational epidemiological studies can be further subdivided into cohort studies (follow-up studies), case control studies, cross-sectional studies (prevalence studies), and ecological studies (correlation studies or studies with aggregated data).
In contrast, studies with only descriptive evaluation are restricted to a simple depiction of the frequency (incidence and prevalence) and distribution of a disease within a population. The objective of the description may also be the regular recording of information (monitoring, surveillance). Registry data are also suited for the description of prevalence and incidence; for example, they are used for national health reports in Germany.
In the simplest case, cohort studies involve the observation of two healthy groups of subjects over time. One group is exposed to a specific substance (for example, workers in a chemical factory) and the other is not exposed. It is recorded prospectively (into the future) how often a specific disease (such as lung cancer) occurs in the two groups ( figure 2a ). The incidence for the occurrence of the disease can be determined for both groups. Moreover, the relative risk (quotient of the incidence rates) is a very important statistical parameter which can be calculated in cohort studies. For rare types of exposure, the general population can be used as controls ( e6 ). All evaluations naturally consider the age and gender distributions in the corresponding cohorts. The objective of cohort studies is to record detailed information on the exposure and on confounding factors, such as the duration of employment, the maximum and the cumulated exposure. One well known cohort study is the British Doctors Study, which prospectively examined the effect of smoking on mortality among British doctors over a period of decades ( e7 ). Cohort studies are well suited for detecting causal connections between exposure and the development of disease. On the other hand, cohort studies often demand a great deal of time, organization, and money. So-called historical cohort studies represent a special case. In this case, all data on exposure and effect (illness) are already available at the start of the study and are analyzed retrospectively. For example, studies of this sort are used to investigate occupational forms of cancer. They are usually cheaper ( 16 ).
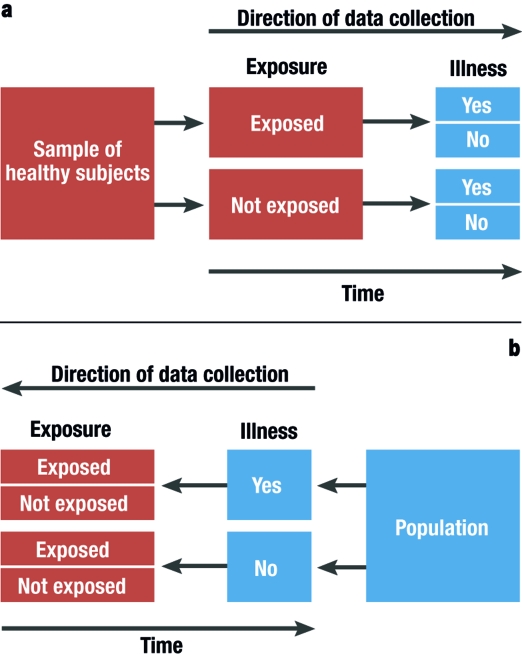
Graphical depiction of a prospective cohort study (simplest case [2a]) and a retrospective case control study (2b)
In case control studies, cases are compared with controls. Cases are persons who fall ill from the disease in question. Controls are persons who are not ill, but are otherwise comparable to the cases. A retrospective analysis is performed to establish to what extent persons in the case and control groups were exposed ( figure 2b ). Possible exposure factors include smoking, nutrition and pollutant load. Care should be taken that the intensity and duration of the exposure is analyzed as carefully and in as detailed a manner as possible. If it is observed that ill people are more often exposed than healthy people, it may be concluded that there is a link between the illness and the risk factor. In case control studies, the most important statistical parameter is the odds ratio. Case control studies usually require less time and fewer resources than cohort studies ( 16 ). The disadvantage of case control studies is that the incidence rate (rate of new cases) cannot be calculated. There is also a great risk of bias from the selection of the study population ("selection bias") and from faulty recall ("recall bias") (see too the article "Avoiding Bias in Observational Studies"). Table 1 presents an overview of possible types of epidemiological study ( e8 ). Table 2 summarizes the advantages and disadvantages of observational studies ( 16 ).
Table 1. Specially well suited study types for epidemiological investigations (taken from [ e8 ]).
Table 2. advantages and disadvantages of observational studies (taken from [ 16 ])*..
1 = slight; 2 = moderate; 3 = high; N/A, not applicable.
*Individual cases may deviate from this pattern.
Selecting the correct study type is an important aspect of study design (see "Study Design in Medical Research" in volume 11/2009). However, the scientific questions can only be correctly answered if the study is planned and performed at a qualitatively high level ( e9 ). It is very important to consider or even eliminate possible interfering factors (or confounders), as otherwise the result cannot be adequately interpreted. Confounders are characteristics which influence the target parameters. Although this influence is not of primary interest, it can interfere with the connection between the target parameter and the factors that are of interest. The influence of confounders can be minimized or eliminated by standardizing the procedure, stratification ( 18 ), or adjustment ( 19 ).
The decision as to which study type is suitable to answer a specific primary research question must be based not only on scientific considerations, but also on issues related to resources (personnel and finances), hospital capacity, and practicability. Many epidemiological studies can only be implemented if there is access to registry data. The demands for planning, implementation, and statistical evaluation for observational studies should be just as high for observational studies as for experimental studies. There are particularly strict requirements, with legally based regulations (such as the Medicines Act and Good Clinical Practice), for the planning, implementation, and evaluation of clinical studies. A study protocol must be prepared for both interventional and noninterventional studies ( 6 , 13 ). The study protocol must contain information on the conditions, question to be answered (objective), the methods of measurement, the implementation, organization, study population, data management, case number planning, the biometric evaluation, and the clinical relevance of the question to be answered ( 13 ).
Important and justified ethical considerations may restrict studies with optimal scientific and statistical features. A randomized intervention study under strictly controlled conditions of the effect of exposure to harmful factors (such as smoking, radiation, or a fatty diet) is not possible and not permissible for ethical reasons. Observational studies are a possible alternative to interventional studies, even though observational studies are less reliable and less easy to control ( 17 ).
A medical study should always be published in a peer reviewed journal. Depending on the study type, there are recommendations and checklists for presenting the results. For example, these may include a description of the population, the procedure for missing values and confounders, and information on statistical parameters. Recommendations and guidelines are available for clinical studies ( 14 , 20 , e10 , e11 ), for diagnostic studies ( 21 , 22 , e12 ), and for epidemiological studies ( 23 , e13 ). Since 2004, the WHO has demanded that studies should be registered in a public registry, such as www.controlled-trials.com or www.clinicaltrials.gov . This demand is supported by the International Committee of Medical Journal Editors (ICMJE) ( 24 ), which specifies that the registration of the study before inclusion of the first subject is an essential condition for the publication of the study results ( e14 ).
When specifying the study type and study design for medical studies, it is essential to collaborate with an experienced biometrician. The quality and reliability of the study can be decisively improved if all important details are planned together ( 12 , 25 ).
Acknowledgments
Translated from the original German by Rodney A. Yeates, M.A., Ph.D.
Conflict of interest statement
The authors declare that there is no conflict of interest in the sense of the International Committee of Medical Journal Editors.
- 1. Bortz J, Döring N. Forschungsmethoden und Evaluation. Springer: Berlin, Heidelberg, New York; 2002. pp. 39–84. [ Google Scholar ]
- 2. Bortz J, Döring N. Forschungsmethoden und Evaluation. Berlin, Heidelberg, New York: Springer; 2002. 37 pp. [ Google Scholar ]
- 3. Fletcher RH, Fletcher SW. Klinische Epidemiologie. Grundlagen und Anwendung. Bern: Huber; 2007. pp. 1–327. [ Google Scholar ]
- 4. Altman DG. Practical statistics for medical research. 1. Aufl. Boca Raton, London, New York, Washington D.C.: Chapman & Hall; 1991. pp. 1–499. [ Google Scholar ]
- 5. Schumacher M, Schulgen G. Methodik klinischer Studien. 2. Aufl. Berlin, Heidelberg, New York: Springer; 2007. pp. 1–436. [ Google Scholar ]
- 6. Machin D, Campbell MJ, Fayers PM, Pinol APY. Sample size tables for clinical studies. 2. Aufl. Oxford, London, Berlin: Blackwell Science Ltd.; 1987. pp. 1–303. [ Google Scholar ]
- 7. Randomization.com: Welcome to randomization.com. http://www.randomization.com/ ; letzte Version: 16. 7. 2008
- 8. Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27:365–375. doi: 10.1016/0021-9681(74)90015-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Altman DG. Randomisation: potential for reducing bias. BMJ. 1991;302:1481–1482. doi: 10.1136/bmj.302.6791.1481. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Fleiss JL. The design and analysis of clinical experiments. New York, Chichester, Brisbane, Toronto, Singapore: John Wiley & Sons; 1986. pp. 120–148. [ Google Scholar ]
- 11. Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Types of epidemiologic studies: clinical trials. 3rd edition. Philadelphia: LIPPINCOTT Williams & Wilkins; 2008. pp. 89–92. [ Google Scholar ]
- 12. Eng J. Sample size estimation: how many individuals should be studied? Radiology. 2003;227:309–313. doi: 10.1148/radiol.2272012051. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Schäfer H, Berger J, Biebler K-E, et al. Empfehlungen für die Erstellung von Studienprotokollen (Studienplänen) für klinische Studien. Informatik, Biometrie und Epidemiologie in Medizin und Biologie. 1999;30:141–154. [ Google Scholar ]
- 14. Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med. 2001;134:657–662. doi: 10.7326/0003-4819-134-8-200104170-00011. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Machin D, Campbell MJ. Design of studies for medical research. Chichester: Wiley; 2005. pp. 1–286. [ Google Scholar ]
- 16. Beaglehole R, Bonita R, Kjellström T. Einführung in die Epidemiologie. Bern: Verlag Hans Huber; 1997. pp. 1–240. [ Google Scholar ]
- 17. Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Types of epidemiologic studies. 3rd Edition. Philadelphia: LIPPINCOTT Williams & Wilkins; 2008. pp. 87–99. [ Google Scholar ]
- 18. Fleiss JL. The design and analysis of clinical experiments. New York, Chichester, Brisbane, Toronto, Singapore: John Wiley & Sons; 1986. pp. 149–185. [ Google Scholar ]
- 19. Fleiss JL. The design and analysis of clinical experiments. New York, Chichester, Brisbane, Toronto, Singapore: John Wiley & Sons; 1986. pp. 186–219. [ Google Scholar ]
- 20. Moher D, Schulz KF, Altman DG. Das CONSORT-Statement: Überarbeitete Empfehlungen zur Qualitätsverbesserung von Reports randomisierter Studien im Parallel-Design. Dtsch Med Wochenschr. 2004;129:16–20. doi: 10.1007/s00482-004-0380-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Clin Chem. 2003;49:1–6. doi: 10.1373/49.1.1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Wald N, Cuckle H. Reporting the assessment of screening and diagnostic tests. Br J Obstet Gynaecol. 1989;96:389–396. doi: 10.1111/j.1471-0528.1989.tb02411.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. International Committee of Medical Journals (ICMJE) Clinical trial registration: a statement from the International Committee of Medical Journal Editors. http://www.icmje.org/clin_trial.pdf ; letzte Version: 22.05.2007
- 25. Altman DG, Gore SM, Gardner MJ, Pocock SJ. Statistical guidelines for contributors to medical journals. Br Med J (Clin Res Ed) 1983;286:1489–1493. doi: 10.1136/bmj.286.6376.1489. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- e1. Neugebauer E, Rothmund M, Lorenz W. The concept, structure and practice of prospective clinical studies. Chirurg. 1989;60:203–213. [ PubMed ] [ Google Scholar ]
- e2. ICH Harmonised Tripartite Guideline. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH); 2008. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- e3. ICH 6: Good Clinical Practice. International Conference on Harmonization; London UK. 1996. adopted by CPMP July 1996 (CPMP/ICH/135/95) [ Google Scholar ]
- e4. ICH 9: Statisticlal Principles for Clinical Trials. International Conference on Harmonization; London UK. 1998. adopted by CPMP July 1998 (CPMP/ICH/363/96) [ Google Scholar ]
- e5. ICH 10: Choice of control group and related issues in clinical trails. International Conference on Harmonization; London UK. 2000. adopted by CPMP July 2000 (CPMP/ICH/363/96) [ PubMed ] [ Google Scholar ]
- e6. Blettner M, Zeeb H, Auvinen A, et al. Mortality from cancer and other causes among male airline cockpit crew in Europe. Int J Cancer. 2003;106:946–952. doi: 10.1002/ijc.11328. [ DOI ] [ PubMed ] [ Google Scholar ]
- e7. Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519–1527. doi: 10.1136/bmj.38142.554479.AE. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- e8. Blettner M, Heuer C, Razum O. Critical reading of epidemiological papers. A guide. Eur J Public Health. 2001;11:97–101. doi: 10.1093/eurpub/11.1.97. [ DOI ] [ PubMed ] [ Google Scholar ]
- e9. Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ. 2001;323:42–46. doi: 10.1136/bmj.323.7303.42. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- e10. Begg C, Cho M, Eastwood S, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276:637–639. doi: 10.1001/jama.276.8.637. [ DOI ] [ PubMed ] [ Google Scholar ]
- e11. Novack GD. The CONSORT statement for publication of controlled clinical trials. Ocul Surf. 2004;2:45–46. doi: 10.1016/s1542-0124(12)70023-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- e12. Bossuyt PM, Reitsma JB, Bruns DE, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Clin Chem. 2003;49:7–18. doi: 10.1373/49.1.7. [ DOI ] [ PubMed ] [ Google Scholar ]
- e13. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology. 2007;18:805–835. doi: 10.1097/EDE.0b013e3181577511. [ DOI ] [ PubMed ] [ Google Scholar ]
- e14. DeAngelis CD, Razen JM, Frizelle FA, et al. Is this clinical trial fully registered: a statement from the International Committee of Medical Journal Editors. JAMA. 2005;293:2908–2917. doi: 10.1001/jama.293.23.jed50037. [ DOI ] [ PubMed ] [ Google Scholar ]
- e15. Altman DG, Gore SM, Gardner MJ, Pocock SJ. Statistical guidelines for contributors to medical journals. Br Med J (Clin Res Ed) 1983;286:1489–1493. doi: 10.1136/bmj.286.6376.1489. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (238.4 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

COMMENTS
FOMAT Medical Research is an Integrated Research Organization (IRO), a subset of a Site Management Organization (SMO), focused on innovating healthcare through diversity. We offer a wide range of solutions and services for Sponsors, Contract Research Organizations (CROs), and Sites. (805) 483 1185. Search. English. Español.
FOMAT Medical Research, headquartered in Oxnard, California, is an Integrated Research Organization (IRO) focused on innovating healthcare with our high-quality services. With over 10 years of experience in participating in Phase 1 through Phase 4 clinical trials in a wide variety of therapeutic areas, we rely on a highly experienced clinical ...
A common way to format research papers is to follow the IMRAD format. This dictates the structure of your paper in the following order: Introduction, Methods, Results, and Discussion. The outline is just the basic structure of your paper. Don't worry if you have to rearrange a few times to get it right.
More than 50% of the resident doctors have the knowledge of p value and protocol writing. 63% students are strongly agreed that research in medical field is important while only 19% students are ...
The International Committee of Medical Journal Editors (ICMJE) offers guidance to authors in its publication Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals (ICMJE Recommendations), which was formerly the Uniform Requirements for Manuscripts.The recommended style for references is based on the National Information Standards Organization ...
The text of articles reporting original research is usually divided into Introduction, Methods, Results, and Discussion sections. This so-called "IMRAD" structure is not an arbitrary publication format but a reflection of the process of scientific discovery. Articles often need subheadings within these sections to further organize their ...
medical journal editors that meets annually to work on the Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical ... research article format (data and PPI statements) • However there is a logic and simplicity that makes the IMRaD structure relevant today.
Classification of different study types *1, sometimes known as experimental research; *2, analogous term: interventional; *3, analogous term: noninterventional or nonexperimental. This scheme is intended to classify the study types as clearly as possible. In the interests of clarity, we have excluded clinical epidemiology — a subject which borders on both clinical and epidemiological ...
Structure of a medical research paper: key content elements, writing tips and examples of reporting guidelines from the EQUATOR website ... - Study design including planned sample size - Interventions (or exposures) - Outcomes (variables) ... Research recommendations Conclusions This section is not always presented separately in a research
What kinds of structures are used? Standardized formats for structured abstracts have been defined for original research studies, review articles and clinical practice guidelines (1,2).The IMRAD format (INTRODUCTION, METHODS, RESULTS, and DISCUSSION), a defacto standard that reflects the process of scientific discovery (), is commonly used as a structure for journal abstracts (4,5).