Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals

Systemic lupus erythematosus articles from across Nature Portfolio
Systemic lupus erythematosus is a potentially fatal autoimmune disease that is characterized by wide-spread inflammation and tissue damage that can affect any part of the body. Commonly affected sites include the liver, kidneys, blood vessels, skin, heart and joints. Patients are treated with immunosuppressive drugs.
The emergence of SLE-causing UNC93B1 variants in 2024
During the past year, four studies have reported ten mutations in UNC93B1 , which encodes the Toll-like receptor (TLR) chaperone protein UNC93B1. All variants increased TLR7 and TLR8 signalling and caused systemic lupus erythematosus in young individuals, and highlight the therapeutic potential of targeting TLR7 and TLR8 in this disease.
- George C. Tsokos
Related Subjects
- Lupus nephritis
Latest Research and Reviews
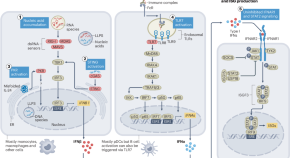
Emerging concepts and treatments in autoinflammatory interferonopathies and monogenic systemic lupus erythematosus
This Review provides a comprehensive update on dysregulated type I interferon production and signalling in autoinflammatory interferonopathies, monogenic systemic lupus erythematosus and conditions that present with broad immune dysregulation and interferon signatures. The authors provide a classification for autoinflammatory interferonopathies based on disease mechanisms of increased type I interferon production and signalling and overlapping clinical phenotypes.
- Raphaela Goldbach-Mansky
- Sara Alehashemi
- Adriana A. de Jesus
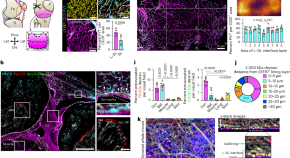
Macrophages and nociceptor neurons form a sentinel unit around fenestrated capillaries to defend the synovium from circulating immune challenge
Why joints are highly responsive to systemic inflammation is unknown. Hasegawa et al. sought to address this question, developing a whole-mount imaging system of the entire synovium to profile the vascular, neuronal and immune components.
- Tetsuo Hasegawa
- Colin Y. C. Lee
- Menna R. Clatworthy
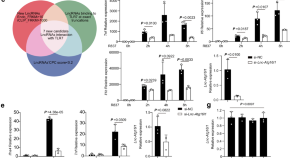
Promotion of TLR7-MyD88-dependent inflammation and autoimmunity in mice through stem-loop changes in Lnc-Atg16l1
TLR7 has been shown to promote inflammatory autoimmunity. Here the authors investigate whether changes in RNA structure affect TLR signaling and show that a TLR7 binding lncRNA Lnc-Atg16l1 promotes development of autoimmunity in the mouse SLE model through enhancement of TLR7-MyD88 interactions.
- Zongheng Yang
Machine learning identifies cytokine signatures of disease severity and autoantibody profiles in systemic lupus erythematosus – a pilot study
- Sarit Sekhar Pattanaik
- Bidyut Kumar Das
- Ratnadeep Mukherjee
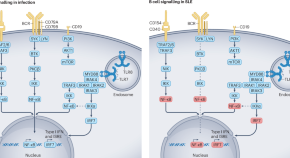
The essential roles of memory B cells in the pathogenesis of systemic lupus erythematosus
BCR-independent memory B cell reactivation via TLR7 or TLR8 activation, type I interferon production, immune complex formation and T helper cell signalling is central in SLE pathogenesis. Dörner and Lipsky discuss the potential of targeting these pathways to eliminate autoreactive memory B cells and plasma cells in SLE.
- Thomas Dörner
- Peter E. Lipsky
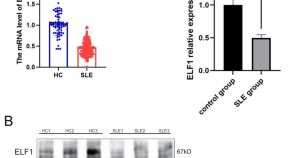
ELF1 serves as a potential biomarker for the disease activity and renal involvement in systemic lupus erythematosus
- Yukun Zhang
- Minglong Cai
- Yujun Sheng
News and Comment
Mito + rbcs prompt ifn and il-1β release by sle monocytes.
In a subset of monocytes in systemic lupus erythematosus, internalization of mitochondria-retaining red blood cells leads to type I interferon-mediated IL-1β release.
- Holly Webster
Autoreactive T cells target neoself-antigens in lupus
Downregulation of invariant chain causes autoreactive t cell expansion.
Neoself-antigens presented by MHC-II molecules in the absence of invariant chain drive clonal expansion of autoreactive T cells in SLE.
- Maria Papatriantafyllou

Interferon disrupts immune and tissue homeostasis in SLE via CXCL13
Type I interferon has a crucial role in the immunopathogenesis of systemic lupus erythematosus (SLE). Analysis of CD4 + T cells from individuals with SLE now shows that type I interferon intervenes with the transcriptional regulators AHR and JUN to downregulate expression of IL-22, which promotes tissue regeneration, and upregulate the expression of CXCL13, which supports lymphoid structure formation.
- Mehrdad Pazhouhandeh
Is X chromosome inactivation a cause or effect of SLE?
Autoimmune diseases such as systemic lupus erythematosus preferentially affect women, and multiple hypotheses are under investigation to elucidate this phenomenon. Emerging research suggests that multiple pathophysiological mechanisms and pathways are likely involved, including several that involve the X chromosome, but is skewing of X chromosome inactivation one of them?
- R. Hal Scofield
- Valerie M. Lewis
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Current Status of the Evaluation and Management of Lupus Patients and Future Prospects
Peter e lipsky.
- Author information
- Article notes
- Copyright and License information
Edited by: Chris Wincup, University College London, United Kingdom
Reviewed by: Matteo Piga, University of Cagliari, Italy; Dafna Gladman, University Health Network, Canada; David Isenberg, University College London, United Kingdom
*Correspondence: Sule Yavuz [email protected] orcid.org/0000-0001-5053-6426
This article was submitted to Rheumatology, a section of the journal Frontiers in Medicine
Received 2021 Mar 18; Accepted 2021 Apr 27; Collection date 2021.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
The vastly diverse nature of systemic lupus erythematosus (SLE) poses great challenges to clinicians and patients, as well as to research and drug development efforts. Precise management of lupus patients would be advanced by the ability to identify specific abnormalities operative in individual patients at the time of encounter with the clinician. Advances in new technologies and bioinformatics have greatly improved the understanding of the pathophysiology of SLE. Recent research has focused on the discovery and classification of sensitive and specific markers that could aid early accurate diagnosis, better monitoring of disease and identification of appropriate therapy choices based on specific dysregulated molecular pathways. Here, we summarize some of the advances and discuss the challenges in moving toward precise patient-centric management modalities in SLE.
Keywords: systemic lupus erythematosus, lupus nephritis, transcriptomic (RNA-Seq), lupus genetics, lupus treatments
Substantial diversity in clinical pictures of SLE is likely the reflection of the underlying molecular heterogeneity.
Measuring disease activity and predicting a flare timely in SLE need instruments that are reliable, practical, and sensitive to capture change between mild and moderate DA.
Integrating advances in new technologies and bioinformatics to clinics may yield more clinically useful tools for better patient care and innovative trial designs.
Patient-centric approach aims at individualized profiling of each patient to assess the main dysregulated pathway (s) and offer the best adequate treatment by predicting the risk of damage in SLE.
Introduction
Systemic lupus erythematosus (SLE) is a very challenging disease for physicians. Given the considerable heterogeneity between individuals as well as within the same individual over time, several aspects of lupus remain uncertain and create substantial unmet medical need. Some of these include early diagnosis, accurate measurement of disease activity, prediction of disease course and flares and tailoring treatment based on the specific patient's need.
Research, improvements in patient care, and the use of immunosuppressives have changed the course of SLE. Survival has improved dramatically over the past 5 decades from a 5-year survival of 50% to a 15-year survival of 85% with current medical regimens ( 1 , 2 ). Despite expanding knowledge, further improvement in survival has mostly stalled, and the rate of progression to renal failure has not improved. Mortality still remains approximately double that of the age-matched general population, with the greatest increase in relative risk in the youngest patients ( 3 – 5 ). Notably, the chance of being dead at the age of 35 for a patient diagnosed with SLE at the age of 20 is one in seven. Moreover, the morbidity linked to the disease itself or side effects of medications, especially damage related to long-term use of glucocorticoids, remains an unresolved problem ( 6 , 7 ). The outlook for patients with lupus nephritis has not dramatically changed over the last years, as 10% of patients still progress to end-stage renal disease ( 8 ).
The link between disease activity, damage, and mortality has been established ( 9 , 10 ). However, the reproducibility of disease activity is less well-documented, and the use of established instruments is not commonplace in clinical practice. Measuring disease activity accurately and in a timely manner is essential not only for better patient care but also for the success of clinical trials. However, currently available instruments are problematic. An optimal tool needs to be reliable, practical, and sensitive to capture change longitudinally. Importantly, for widespread adoption, it must be logical to a health care professional (HCP), incorporate elements of patient concerns, and impose a minimal administrative burden. Additional challenges arise when considering well-recognized ethnic disparities, with the highest SLE disease burden in non-white populations. Observed disparities also exist at the genetic and cellular level ( 11 – 14 ); patient ancestry impacts gene expression patterns significantly in SLE as well as the dominant molecular pathways active in disease pathogenesis and response to therapeutics also appears to be affected ( 15 , 16 ). However, the majority of clinical trials consist disproportionately of more European Ancestry (Caucasian) patients ( 17 ). Moreover, in clinical practice, ancestral diversity is rarely considered when treatments are prescribed, even though there is considerable evidence of differential responsiveness to both standard of care and off-label medications ( 18 , 19 ).
Clinical trials have also been affected by the complexity of characterizing SLE patients precisely. The results of clinical trials in SLE have been largely disappointing compared to successes in rheumatoid arthritis, psoriatic arthritis and spondylitis. Only two agents, Belimumab, a monoclonal antibody that binds BAFF (TNFSF13B), and very recently, Voclosporin have been approved for SLE and LN, respectively, since 1959 ( 16 , 20 ). The possible reasons are discussed in detail elsewhere ( 21 ), but a hint may be hidden in the trials showing that up to 30% of established SLE patients screened for a new therapy are anti-nuclear antibody (ANA) negative. This observation is surprising because ANA was previously thought to be persistently present in most if not all SLE patients ( 22 , 23 ), and spurs several questions around ANA, such as the possibility of ANA positive SLE patients (so called immune-active) may respond to some therapies differently from ANA negative patients. This possibility raises the interesting question as to whether ANA positivity might be a theragnostic in SLE ( 24 ).
Recently, several immunologic discoveries and genetic association studies in SLE have immensely improved understanding of the pathophysiological mechanisms underlying SLE molecular and clinical heterogeneity. Defining the molecular traits and developing innovative tools that integrate molecular pathways and clinic manifestations may transform current clinical approaches to SLE and provide the basis for patient-centric precision medicine in SLE.
Early Diagnosis
Diagnostic delays and misdiagnosis are common in SLE. A survey of more than 2,500 UK lupus patients in 2014 showed that the mean time between patients' first awareness of SLE symptoms and actual diagnosis was 6.4 years and half of the patients reported that they had been misdiagnosed initially ( 25 ). Diagnosis in SLE relies on the physician's clinical judgment based on a combination of clinical signs/symptoms and available clinical tests (most frequently ANAs). Early diagnosis and effective treatment may prevent long-term complications that cause increased morbidity and mortality. Given the considerable heterogeneity in most disorders in rheumatology, including SLE, the development and validation of diagnostic criteria can be quite challenging. As a result, there are only a few validated diagnostic criteria in rheumatology ( 26 ). Most efforts have been directed toward developing more precise classification criteria that aim to assemble cohorts that are representative of the majority with disease for clinical research. In practice, on the other hand, physicians often use classification criteria to diagnose complex diseases like SLE ( 27 ). Ideally, when the sensitivity and specificity ~100%, it is possible to employ the terms interchangeably, although this is not recommended. Whereas, classification criteria tend to include features phenotypically more prevalent in relevant diseases, the use of classification criteria tends to leave a group of patients with an incomplete set of criteria or uncommon features misclassified. The development process of recent ACR/EULAR SLE classification criteria ( 28 – 30 ) took into consideration these issues, such as applicability to early or new onset lupus without compromising specificity and focusing on true autoimmune disease. In this context, the 2019 EULAR/ACR criteria performed well in early disease, defined as 1 to <3 years of disease duration, with a sensitivity better than the former ACR criteria (97 vs. 81%) and a specificity better than the 2012 SLICC criteria (96 vs. 88%) ( 31 ).
Data has shown that autoantibodies, such as ANA, appear in the blood as early as 9.4 years (mean 3.3 years) before the clinical onset of SLE and the pattern evolves, reflective of the disease process. Closer to the onset of overt disease, more specific autoantibodies (e.g., anti-dsDNA, anti-Sm) are detected ( 32 ). Given the low specificity of ANA for SLE, screening patients with non-specific symptoms becomes a burden for many rheumatology clinics across the globe, as it is positive in up to 20% of individuals without SLE. This is also the rationale behind the 200-antigen immune-chip assay, SLE-key (ImmunArray, Richmond, VA, USA), which claimed a sensitivity of 94% in ruling out the non-diseased subjects, but is no longer available. Another assay detecting cell-bound complement activation products (Exagen, Vista, CA, USA) , which outperforms anti-dsDNA by up to 48%, has been in use for assisting physician in diagnosing SLE for some time ( 33 ).
SLE is heavily influenced by genetics, and so far, ~100 genetic susceptibility loci have been identified ( 14 ). Recent advances have prompted the possibility of utilizing genetic risk scores (GRS) as tools for predicting disease susceptibility and outcome in SLE ( 34 , 35 ). GRS are numeric scores that combine a large number of disease-associated genetic variants that are weighted by SLE risk odds ratios and reflect the disease associated genetic load in an individual patient ( 36 ). Knevel et al. ( 37 ) have developed a GRS (G-PROB) using genome-wide significant variants ( p ≤ 5 × 10 −8 ) from previously published genome-wide association studies (GWAS), and tested its potential as a diagnostic tool in a set of patients with inflammatory arthritis (rheumatoid arthritis, systemic lupus erythematosus, spondyloarthropathy, psoriatic arthritis, and gout). Coupled with good discriminatory capacity (area under the curve, AUC: 0.69–0.84), it could single out a likely diagnosis for 45% of patients with a positive predictive value of 0.64, that could be further improved with serologic data. Despite only being tested in Caucasian cohorts, the results of this study demonstrate the potential clinical utility of the GRS for diagnosis, especially when incorporating serologic findings, such as ANA, and, possibly, clinical manifestations.
Transcriptomic analysis might also contribute to diagnosis. Based on a meta-analysis of 40 independent publicly available gene-expression studies containing 7,471 transcriptomic profiles, Haynes et al. identified a core gene set (93-gene signature, SLE MetaSignature) that is dysregulated in patients with SLE and distinguishes SLE from other relevant rheumatic disorder and infections ( 38 ). They further validated the SLE MetaSignature in a prospective study comprising patients with juvenile onset SLE, juvenile idiopathic arthritis, and healthy subjects. This study demonstrates the potential value of the integration of gene-expression studies into the clinic as a means to improve the diagnosis of SLE.
Challenges in Established Disease
Disease activity.
Loss of tolerance to nucleic acids and sustained autoantibody production along with an imbalance between the production of apoptotic material and its disposal are the key features in SLE pathogenesis. In addition to the complex genetic background in the majority of the patients, epigenetic, and environmental factors may contribute to the disease and influence the occurrence of flares. The diverse clinical picture of SLE likely reflects underlying molecular heterogeneity coupled with unpredictable fluctuations in disease activity perhaps reflective of environmental triggers, which leads to organ damage accrual, challenges clinicians, and hampers clinical trials. Different instruments have been developed to measure general disease activity, of which the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) and the British Isles Lupus Assessment Group (BILAG) are predominantly used in clinical trials ( 39 ). However, none of these instruments is perfect and neither is routinely used in clinical practice ( Table 1 ). Traditional biomarkers, such as complement and anti-dsDNA, are commonly used to detect a flare; however, fluctuations in serology may not predict a flare as shown in a long term follow-up of a cohort of serologically active but clinically quiescent patients ( 40 ). Moreover, these tests have been studied most in patients with lupus nephritis and their utility in detecting other manifestations of SLE is less clear. More comprehensive tools that integrate novel biomarkers, identified by longitudinal analyses of well-characterized cohorts and the use of new big data analytical techniques, such as machine learning, could be valuable for capturing disease activity and providing timely stratification of patients based on the unified pattern of dysregulated pathways ( 41 , 42 ). Transcriptomic studies have identified distinctive signatures in SLE blood samples that include type I interferons (IFN), as well as myeloid and B cell-related (plasma cell) signatures ( 43 – 46 ). Several studies also showed a correlation between these signatures and disease activity ( 42 – 44 ). Although a robust interferon gene signature (IGS) has been demonstrated in both blood and affected tissues of SLE patients ( 47 ), no apparent correlations have been found between the IGS and disease activity in longitudinal studies ( 46 , 48 , 49 ). Both type I and type II IFNs (IFNγ) contribute to the IGS. However, only modest differences in enrichment of genes downstream of specific IFNs in cells and tissue have been demonstrated in SLE ( 46 ). The aforementioned study also uncovered that the prolonged IGS in monocytes, even when disease activity is low, is likely the reason that the IGS is not useful in the detection of disease activity accurately.
Some of the potential urinary biomarkers.
TWEAK, TNF-like weak inducer of apoptosis; BAFF, B cell activating factor; TGF-β, transforming growth factor beta; CCL2, chemokine (C-C motif) ligand 2/MCP-1, monocyte chemoattractant protein-1; CXCL10, C-X-C motif chemokine 10/IP-10, Interferon gamma-induced protein 10; CXCL4, C-X-C motif chemokine 4; VCAM-1, vascular cell adhesion molecule 1; ALCAM, activated leucocyte cell adhesion molecule; NGAL, neutrophil gelatinase-associated lipocallin .
Epigenetic mechanisms have also emerged as important factors in SLE pathogenesis ( 48 , 49 ). Aberrant changes in DNA methylation, histone modification, and non-coding RNAs, induced by environmental influences or regulated by genetic factors, can alter gene expression. Epigenetic modifications may vary depending on the location, cell type, ethnicity, response to therapy, and the course of the disease. Studies of DNA methylation, which is the most widely studied epigenetic marker, have consistently shown demethylation at interferon-regulated genes across all the investigated cell-types in SLE, independent of disease activity ( 50 , 51 ). Notably, altered DNA methylation of particular genes involved in T cell biology and genes regulated by interferon seems to be associated with lupus disease activity and show promise as markers for disease monitoring ( 52 , 53 ). So far, most epigenetic studies in lupus have been cross-sectional, and epigenetic changes over time and their relation with disease activity have been unclear. In this respect, a recent longitudinal study assessing the DNA methylome of neutrophils across ancestries has reported two differentially methylated CpG sites unique to African-American patients that are associated with lupus disease activity ( 54 ). MicroRNAs (miR), small non-coding RNA molecules, are closely linked to DNA methylation and thus to epigenetic changes that regulate the expression of multiple genes. Serum miR levels change during active or inactive disease states and may serve as a disease biomarker. In fact, miR-146, miR-21, and miR-148a correlate with lupus disease activity and have been proposed as biomarkers ( 55 , 56 ).
Lupus Nephritis and Application of Cutting Edge Technologies
Lupus nephritis (LN) is a common feature of SLE that causes higher morbidity and mortality ( 8 , 57 ). As LN is usually asymptomatic, the common practice is the regular screening of urine samples of SLE patients for proteinuria or active sediment. When either of these exists, a renal biopsy, as the “gold standard,” is frequently employed to confirm diagnosis and guide treatment. However, the current histopathological classification system has some limitations. It focuses mainly on glomerular lesions and interstitial lesions are incompletely addressed. This is a deficit because recent evidence suggests that interstitial involvement is predictive of progression to renal failure ( 58 ).More importantly, the underlying molecular pathways cannot be assessed by routine histology or immunofluorescence studies or even electron microscopy. It should be emphasized that only 30% of LN patients achieve a complete remission with the current treatment paradigm.
Leveraging powerful technologies, such as single-cell transcriptomics, could provide new insights into the diverse mechanisms involved in the pathogenesis of tissue injury. Information from these studies may advance current understanding of the complex interaction between immune and resident cells in the kidney as well as identify drivers of renal flares; the result could be novel biomarkers as well as the development of clinically meaningful user-friendly metrics for prognosis and treatment response. In this context, single-cell transcriptomic analyses show different clusters of myeloid, T, and B cell subtypes, where NK and CD8 + cytotoxic T cells are major proliferating populations in LN kidney samples ( 59 ). By contrast, few B cells are detected in healthy kidneys by either flow cytometry or single-cell mRNA sequencing, whereas resident macrophages and memory CD4 + T cells are the dominating cell populations. Neutrophil signatures in the blood are also associated with active LN ( 43 , 60 ), whereas single-cell transcriptomic profiles of neutrophils have not yet been identified in the LN kidney. Kidney epithelial cells also contribute to disease progression in LN, as demonstrated by profiling unselected kidney cells in a parallel study. Tubular epithelial cells differentially express the IGS and fibrosis-associated genes are found in those samples from patients who had an inadequate response to treatment ( 61 ). Despite these interesting results, it is still unclear how different disease states, background treatment, and ancestry influence the composition of cells and their gene expression. Although there are well-recognized limitations of single-cell RNA sequencing, these initial studies provide a framework for understanding the immune cell types potentially contributing to LN ( 62 , 63 ). Questions that are more specific, such as whether T and B cells in the kidney are antigen-specific or spatial relationships among detected cells should also be addressed in future studies.
Changes in lupus kidneys are dynamic, and longitudinal tissue sampling would facilitate tracking disease progression and response to the intervention. However, renal biopsy is not entirely risk-free. Conventionally used biomarkers such as proteinuria or serologic markers have a limited ability to predict renal prognosis adequately, and persistent proteinuria can be secondary to residual activity or chronic damage. Given that the urine is easily accessible and urinary biomarkers may directly reflect the underlying disease process in the lupus kidney, several candidates have been identified ( Table 2 ). The repertoire of potential urinary biomarkers has been somewhat disappointing, however, as only a limited number has been validated in longitudinal cohorts, few have been tested in multiple ancestries and none has been successfully used in clinical trials ( 64 ). The sources of proteins in the urine are both from circulation and local cells (infiltrating immune or resident) in the kidney, thus potentially rendering urine a valuable source of biomarkers for both systemic and intrinsic kidney diseases. Advances in proteomic techniques allow for screening a large number of proteins simultaneously with the aim of discovering novel biomarkers. A recent high throughput urine proteome study integrated with single-cell transcriptomic of lupus kidneys detected a chemokine profile, inducible by IFN-γ, which is produced mainly by CD8 + T cells in active LN patients ( 65 ). Although this study was limited by the sample size, it is an encouraging example of the potential of urine proteome profiling to predict active immune pathways in the kidney.
Comparison of two commonly used instruments in clinical trials.
BILAG 2004, updated version of British Isles Lupus Assessment Group; Systemic Lupus Erythematosus .
Disease Activity Index 2000; DA, Disease activity .
Outcome Measures
Given the link among disease activity, damage, and mortality, targeting low disease activity (LDA) seems a logical treatment goal in SLE, in which remission, especially durable drug free remission, is difficult to achieve ( 66 ) ( Box 1 ). The Asia Pacific Lupus Collaboration has generated the Lupus Low Disease Activity State (LLDAS) metric for generalized SLE, which has shown to be associated with reduced damage accrual ( 67 – 69 ). Retrospective studies suggest that LLDAS might have value as a outcome measure in clinical trials ( 69 , 70 ). The Lupus Multivariable Outcome Score (LuMOS), a multivariable response score, was developed to address the inherent heterogeneity of SLE patients, which may be responsible for the frequent failure of the responder indices used in clinical trials. LuMOS utilized information collected from different variables using the Study of Belimumab in Subjects with SLE 76-week (BLISS-76) as training dataset and an independent dataset from the BLISS-52 trial as validation ( 71 ).The LuMOS outperformed SRI-4, the original outcome measure, in the BLISS-52 trial ( 72 ) in terms of the effect size. However, data from the RCTs in SLE other than belimumab and different subpopulations or longitudinal studies in well-defined SLE cohorts may be necessary to provide further justification for its performance in comparison to other outcome measures used in clinical trials in SLE.
Box 1. Lupus low disease activity state ( 65 ).
Disease activity
SLEDAI-2K ≤ 4, with no activity in major organ systems (renal, CNS, cardiopulmonary, vasculitis, fever) and no hemolytic anemia or gastrointestinal activity
No new features of lupus disease activity compared with the previous assessment
SELENA-SLEDAI physician global assessment (PGA, scale 0–3) ≤ 1
Immunosuppressive medications
Current prednisolone (or equivalnt) dose ≤ 7.5 mg daily
Well-tolerated standard maintenance doses of immunosuppressive drugs and approved biological agents, excluding investigational drugs SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000.
SELENA-SLEDAI, Safety of Estrogen in Lupus Erythematosus National Assessment-Systemic Lupus Erythematosus Disease Activity Index. PGA, Physician Global Assessment
Organ-specific disease features and their contribution to end-organ damage have been well-described for the kidney, skin, and central nervous system (CNS) ( 73 ). Given the different weighing of clinical manifestations in different outcome measures, two patients with entirely different clinical features may yield the same score. As considerable clinical diversity exists, a significant response detected with one of these tools might not necessarily reflect a meaningful clinical response for clinicians and patients in SLE. Even within the same organ system, for instance, skin involvement, lupus rash may exhibit a different degree of activity between two patients. If one has 5% of the body surface (skin color: faint erythema) covered, whereas the other has 15% (skin color: dark red), nevertheless, both of them get the weighted score of 2 on SLEDAI-2K, rash as a descriptor. For individualized treatment, disease activity and organ involvement need to be assessed objectively and accurately. Should that be the case, it would be essential to ascertain the specific goal and the outcome measure (general SLE, organ-specific, or both) that is most appropriate. To this end, the academic community has developed the Cutaneous Lupus Area and Severity Index (CLASI) to assess skin activity and capture differences, but this has not yet been accepted by regulatory authorities as a validated outcome measure in clinical trials ( 74 ).
As stated above, predicting disease flare accurately and preventing it is an essential task for clinicians as recurrent flares are associated with organ damage accrual and mortality in SLE ( 9 , 75 ). Given its obvious relevance to optimal patient care, measuring flare as an endpoint in clinical trials should also be considered with a physician-friendly carefully devised instrument that could discriminate moderate to mild flares reproducibly ( 41 ).
Patient Satisfaction and Fatigue
Data show that a patient's active participation in her/his healthcare decisions with physicians may improve outcomes and lead to higher patient adherence and satisfaction ( 76 , 77 ). However, there are often gaps between patient's preferences and what physicians think is the best for patient care. Physicians usually take into account a range of clinical and laboratory outcomes to consider SLE treatment satisfactory, whereas patients mostly prioritize manifestations that impact their daily functioning. Fatigue is one of the most common (70–90%) features of SLE and can be both debilitating and difficult to treat, and clearly, represents a major unmet need with substantial impact on the patient's daily functioning and quality of life ( 78 – 80 ). Both the FDA and the European Medicines Agency (EMA) highlight fatigue as being an important patient-reported outcome (PRO). FDA advises using PROs as secondary outcomes in SLE clinical trials; however, no specific instrument for measuring the level of fatigue has been recommended.
The causes of fatigue in lupus are poorly understood and several possible mechanisms are likely to contribute ( Table 3 ). Fatigue is a common (80–90%) dose-dependent side effect of interferon (IFN)-α treatment of HCV or malignancy ( 81 ), and type 1 IFNs are known to play a central role in SLE pathogenesis. As circulating IFN-α may not easily cross the brain blood barrier, it may exert its central effect via other cytokines that are induced in SLE, such as TNF, IL-1, and IL-6, all of which are implicated in the pathogenesis of chronic fatigue as well ( 81 , 82 ). However, attribution of fatigue to an immunologic process directly associated with immune alterations in SLE has been extremely difficult. This may be related to underpowered studies or the lack of sufficient tools to capture changes in fatigue levels. In a recent study comprising 426 SLE patients, a close correlation was found between circulating antibodies to the NR2 subunit of the N-methyl-D-aspartate receptor (NMDAR) and the severity of fatigue in addition to the clinical disease activity index (Systemic Lupus Erythematosus Disease Activity Index 2000) and anti-double stranded DNA antibodies, independently of the presence of neuropsychiatric lupus manifestations ( 83 ). The presence of anti-NR2 antibodies may be a helpful diagnostic tool in the evaluation and therapeutic management of fatigue. The BLISS trials investigating the use of Belimumab in SLE showed improvement of fatigue scores measured by the FACIT-fatigue (FACIT-F) scale ( 84 ). Interestingly, using belimumab for at least 6 months affected the levels of anti-NR2 antibodies along with fatigue severity ( 34 ).
Possible contributors of fatigue.
On the other hand, fatigue could also be a result of corticosteroid withdrawal. In a recent RCT comparing two different administrations of the same daily dose of prednisone, the delayed-release in the evening compared to the immediate-release in the morning in patients previously receiving the same dose of corticosteroids, a significant improvement in fatigue levels was noted in both arms, compared to that present at entry. This may imply that one component of fatigue may be linked to the difference between the actual administered dose of prednisone by the patient and what is being prescribed ( 85 ).

Toward Patient-Centric Clinical Trials in SLE
The introduction of biological and targeted disease-modifying drugs into clinical practice has revolutionized the outlook of patients with RA, psoriatic arthritis, and ankylosing spondylitis. Unfortunately, SLE lags far behind these achievements in other inflammatory arthropathies, and belimumab is the only biologic approved for the treatment of SLE and LN in the last 60 years ( 86 ). Failure to achieve primary end-points of many study drugs in different clinical trials in SLE has led to several treatment guides that contain recommendations based on clinical experience or eminence-based rather than evidence-based ones for physicians ( 87 – 89 ). The lack of biomarkers to forecast treatment response to individual drugs limits the ability to prevent patients from cycling through a few approved and unapproved drugs and protect them from unnecessary side effects of immunosuppressive drugs or glucocorticoids. So far, a considerable amount of valuable information has been gleaned from post-hoc analyses of these failed trials. Of note, however, because of substantial molecular heterogeneity in SLE, inclusion of patients with molecular subtypes lacking the target of the drug imposes problems when interpreting the trial results. Some of the issues that might lead to the failure of clinical trials in SLE are listed in Box 2 .
Box 2. Some of the problems in SLE drug development.
- ° molecular heterogeneity
- ° African, Asian, Hispanic, and Aboriginal ancestry develop SLE earlier and tend to have higher disease activity and mortality, yet underrepresented in trials
Endpoint definitions
- ° some endpoints can be achieved beyond 52 weeks
Background therapy
Lack of reliable biomarker
Patient for the trial vs. trial for the patient?
More recently, the second of 3 trials of anifrolumab (TULIP-2), an antibody against the type-I interferon receptor, has shown a significant effect on the primary end point of response, according to British Isles Lupus Assessment Group (BILAG) based composite lupus assessment (BICLA) at week 52 ( 90 ). TULIP-2 has also pointed out the challenges that exist in the trial design of SLE for the selection of endpoints, as the TULIP-1 trial did not reach significance for its primary endpoint, the Systemic Lupus Erythematosus Responder Index (SRI-4) ( 72 , 91 ). Both of these instruments were devised during the development of the belimumab and epratuzumab trials, and were expected to provide concordant results ( 92 ). Even these two almost identically designed TULIP trials with conflicting results underline that clinical trials may be benefited from the re-evaluation of the outcome measures that define more clinically relevant endpoints and their integration into trials.
Current randomized controlled trial (RCT) strategies assume that the target population is homogenous, for example, with respect to disease activity or serology. However, substantial biologic heterogeneity and its potential link to diverse clinical phenotypes in SLE have already been underscored in several studies ( 45 , 63 ). Stratifying patients based on underlying molecular pathology is a tantalizing prospect using innovative patient-centric targeted treatment designs that may enhance response rates, as already observed in oncology ( Figure 1 ) ( 93 ). Recent trials with anifrolumab ( 94 ), which blocks type 1 interferon, and ongoing trials for Atacicept (blocks BAFF and APRIL) ( 95 ), demonstrate the possibility of success using approaches that are more precise in the treatment of SLE. Earlier data suggested that the IGS would facilitate better discernment of the SLE patients who might respond to anifrolumab and possibly serve as a biomarker ( 96 ). The results of the TULIP-2 trial did show a greater treatment effect in patients with a high IGS, but not greatly different from those with a low IGS. It is unclear whether this result is related to assay performance, timing, or something else; it certainly needs further exploration. The incorporation of molecular phenotypes into clinical trials in SLE has also been tested using the results of the ADDRESS II study of atacicept. The investigators carried out a cell deconvolution algorithm in the baseline gene expression profiles to confirm the differential treatment effect observed in the ADDRESS II study ( 97 ). In this exploratory study, they showed that patients with high B cell or plasmablast gene expression, especially within the high disease activity group, demonstrated a better atacicept treatment response, which is relevant to the proposed mechanism of action of the drug. Nevertheless, the phase 2 trial failed to meet its primary clinical endpoint.
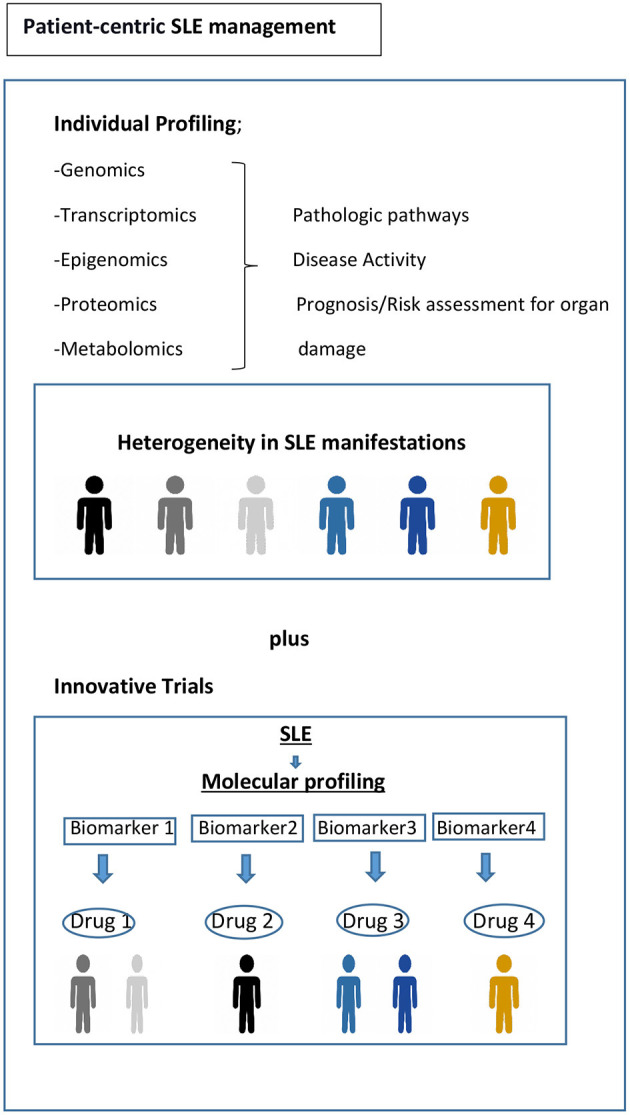
Toward precision medicine. Molecular profiling of patients at time of diagnosis and throughout their disease course would be helpful to assess the drivers of main pathophysiological pathways, to predict organ damage accrual and flares, and to offer optimum treatment and follow-up. Biomarker driven trial designs are proposed here. Adapted from REF ( 93 ).
Other important considerations are the variations in treatment response between ethnicities that have been shown in different studies ( 11 , 18 ). For example, African ancestry (AA) patients with LN may have a more favorable response to rituximab ( 15 ), which is compatible with the gene signature seen in AA patients that is characterized by a perturbed B cell axis ( 12 ). Although rituximab ( 19 ) failed to show a significant effect compared to standard of care in patients with LN in the LUNAR trial, there is consensus that in some patients, B cell deletion by targeting CD20 is beneficial ( 98 , 99 ). In practice, many physicians use rituximab as a last resort for those who failed to respond to the current SOC ( 86 ). A recent study also pointed out the importance of taking into account genetic differences across ancestries that may result in differences in response to drug targets ( 100 ). By utilizing expression quantitative trait loci (eQTL) mapping and a comprehensive systems biology approach for predicting SLE-associated pathways, the investigators identified both common and ancestry specific associations. Whereas, the pathways related to innate immune and myeloid cell functions were enriched in patients of European ancestry (EA), the pathways associated with aberrant B cell activity along with the ER stress and metabolic dysfunction were enriched in patients of AA.
Drug Repurposing in SLE
Drug repurposing analyses can identify a new therapeutic use of a drug based on the ability to revert a pathological gene expression signature in a condition that is not its primary indication in clinical practice ( 101 , 102 ). As a robust alternative to de novo drug discovery, drug repurposing analyses have the advantage of employing many data-driven strategies that integrate multiple sources of data. Given the paucity of licensed therapeutic agents for SLE, these have also been performed in SLE ( 103 , 104 ). The comparison of transcriptomic data from longitudinally followed SLE patients with drug-induced gene signatures from the widely used analytical tool, Connectivity Map Linked User Environment (CLUE) database, revealed differences in drug-induced gene-expression connectivity scores based on the patient subset enriched for neutrophils and lymphocytes ( 104 ). However, further clinical studies are required to determine whether this type of sub-setting of patients can improve the treatment or indicate novel therapies.
The current landscape of research in genetic(s)/omic(s) presents an opportunity for the integration of molecular phenotype and clinical phenotype to address unmet needs in SLE. Recent advances in new technologies and analytical approaches have facilitated the discovery of molecular pathways, potential biomarker candidates, and drug repurposing efforts that may improve classification and treatment of patients with SLE. However, shifting from the current paradigms in patient care and drug development will require the demonstration of the clinical utility of these advances using innovative clinical trials. Despite the challenges, the future for patients with SLE seems propitious. Patient-centric precision medicine has the potential to offer the proper drug(s) for a patient's condition determined by individual molecular phenotype, thereby targeting disease pathophysiology and treatment response. However, achieving this will require consensus within academia, physicians, industry, patient organizations, and regulatory agencies.
Author Contributions
SY and PL wrote the manuscript. All authors read, provided critical review, and accepted the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.682544/full#supplementary-material
- 1. Mak A, Cheung MW, Chiew HJ, Liu Y, Ho RC. Global trend of survival and damage of systemic lupus erythematosus: meta-analysis and meta-regression of observational studies from the 1950s to 2000s. Semin Arthritis Rheum. (2012) 41:830–9. 10.1016/j.semarthrit.2011.11.002 [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Yen EY, Shaheen M, Woo JMP, Mercer N, Li N, McCurdy DK, et al. 46-year trends in systemic lupus erythematosus mortality in the United States, 1968 to 2013: a nationwide population-based study. Ann Intern Med. (2017). 167:777–85. 10.7326/M17-0102 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Bultink IEM, de Vries F, van Vollenhoven RF, Lalmohamed A. Mortality, causes of death and influence of medication use in patients with systemic lupus erythematosus vs matched controls. Rheumatology. (2020) 60:207–16. 10.1093/rheumatology/keaa267 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Tselios K, Gladman DD, Sheane BJ, Su J, Urowitz M. All-cause, cause-specific and age-specific standardised mortality ratios of patients with systemic lupus erythematosus in Ontario, Canada over 43 years (1971-2013). Ann Rheum Dis. (2019) 78:802–6. 10.1136/annrheumdis-2018-214802 [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Jorge AM, Lu N, Zhang Y, Rai SK, Choi HK. Unchanging premature mortality trends in systemic lupus erythematosus: a general population-based study (1999-2014). Rheumatology. (2018) 57:337–44. 10.1093/rheumatology/kex412 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Zonana-Nacach A, Barr SG, Magder LS, Petri M. Damage in systemic lupus erythematosus and its association with corticosteroids. Arthritis Rheum. (2000) 43:1801–8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. van Vollenhoven RF, Petri MA, Cervera R, Roth DA, Ji BN, Kleoudis CS, et al. Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Ann Rheum Dis. (2012) 71:1343–9. 10.1136/annrheumdis-2011-200937 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Tektonidou MG, Dasgupta A, Ward MM. Risk of end-stage renal disease in patients with lupus nephritis, 1971-2015: a systematic review and bayesian meta-analysis. Arthritis Rheumatol. (2016) 68:1432–41. 10.1002/art.39594 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Bruce IN, O'Keeffe AG, Farewell V, Hanly JG, Manzi S, Su L, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis. (2015) 74:1706–13. 10.1136/annrheumdis-2013-205171 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Rahman P, Gladman DD, Urowitz MB, Hallett D, Tam LS. Early damage as measured by the SLICC/ACR damage index is a predictor of mortality in systemic lupus erythematosus. Lupus. (2001) 10:93–6. 10.1191/096120301670679959 [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Menard LC, Habte S, Gonsiorek W, Lee D, Banas D, Holloway DA, et al. B cells from African American lupus patients exhibit an activated phenotype. JCI Insight. (2016) 1:e87310. 10.1172/jci.insight.87310 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Catalina MD, Bachali P, Yeo AE, Geraci NS, Petri MA, Grammer AC, et al. Patient ancestry significantly contributes to molecular heterogeneity of systemic lupus erythematosus. JCI Insight. (2020) 5. 10.1172/jci.insight.140380 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Nédélec Y, Sanz J, Baharian G, Szpiech ZA, Pacis A, Dumaine A, et al. Genetic ancestry and natural selection drive population differences in immune responses to pathogens. Cell. (2016) 167:657–69.e21. 10.1016/j.cell.2016.09.025 [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Langefeld CD, Ainsworth HC, Cunninghame Graham DS, Kelly JA, Comeau ME, Marion MC, et al. Transancestral mapping and genetic load in systemic lupus erythematosus. Nat Commun. (2017) 8:16021. 10.1038/ncomms16021 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. (2010) 62:222–33. 10.1002/art.27233 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzová D, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. (2011) 63:3918–30. 10.1002/art.30613 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Anjorin A, Lipsky P. Engaging African ancestry participants in SLE clinical trials. Lupus Sci Med. (2018) 5:e000297. 10.1136/lupus-2018-000297 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Dooley MA, Hogan S, Jennette C, Falk R. Cyclophosphamide therapy for lupus nephritis: poor renal survival in black Americans. Glomerular disease collaborative network. Kidney Int. (1997) 51:1188–95. 10.1038/ki.1997.162 [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. (2012) 64:1215–26. 10.1002/art.34359 [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Wallace DJ, Stohl W, Furie RA, Lisse JR, McKay JD, Merrill JT, et al. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Rheum. (2009) 61:1168–78. 10.1002/art.24699 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Bruce IN, Gordon C, Merrill JT, Isenberg D. Clinical trials in lupus: what have we learned so far? Rheumatology. (2010) 49:1025–7. 10.1093/rheumatology/kep462 [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Putterman C, Pisetsky DS, Petri M, Caricchio R, Wu AHB, Sanz I, et al. The SLE-key test serological signature: new insights into the course of lupus. Rheumatology. (2018) 57:1632–40. 10.1093/rheumatology/key149 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Pisetsky DS, Spencer DM, Rovin B, Lipsky PE. Role of ANA testing in the classification of patients with systemic lupus erythematosus. Ann Rheum Dis. (2019) 2019:216259. 10.1136/annrheumdis-2019-216259 [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Pisetsky DS, Lipsky PE. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat Rev Rheumatol. (2020) 16:565–79. 10.1038/s41584-020-0480-7 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Morgan C, Bland AR, Maker C, Dunnage J, Bruce IN. Individuals living with lupus: findings from the LUPUS UK Members Survey 2014. Lupus. (2018) 27:681–7. 10.1177/0961203317749746 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Aggarwal R, Ringold S, Khanna D, Neogi T, Johnson SR, Miller A, et al. Distinctions between diagnostic and classification criteria? Arthritis Care Res. (2015) 67:891–7. 10.1002/acr.22583 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Tucker LB. Making the diagnosis of systemic lupus erythematosus in children and adolescents. Lupus. (2007) 16:546–9. 10.1177/0961203307078068 [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. (2019). 78:1151–9. 10.1136/annrheumdis-2019-216700 [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Johnson SR, Khanna D, Daikh D, Cervera R, Costedoat-Chalumeau N, Gladman DD, et al. Use of consensus methodology to determine candidate items for systemic lupus erythematosus classification criteria. J Rheumatol. (2019) 46:721–6. 10.3899/jrheum.180478 [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. (2019). 71:1400–12. 10.1002/art.40930 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Johnson SR, Brinks R, Costenbader KH, Daikh D, Mosca M, Ramsey-Goldman R, et al. Performance of the 2019 EULAR/ACR classification criteria for systemic lupus erythematosus in early disease, across sexes and ethnicities. Ann Rheum Dis. (2020) 79:1333–9. 10.1136/annrheumdis-2020-219314 [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. (2003) 349:1526–33. 10.1056/NEJMoa021933 [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Putterman C, Furie R, Ramsey-Goldman R, Askanase A, Buyon J, Kalunian K, et al. Cell-bound complement activation products in systemic lupus erythematosus: comparison with anti-double-stranded DNA and standard complement measurements. Lupus Sci Med. (2014) 1:e000056. 10.1136/lupus-2014-000056 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Chung SA, Brown EE, Williams AH, Ramos PS, Berthier CC, Bhangale T, et al. Lupus nephritis susceptibility loci in women with systemic lupus erythematosus. J Am Soc Nephrol. (2014) 25:2859–70. 10.1681/ASN.2013050446 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Reid S, Alexsson A, Frodlund M, Morris D, Sandling JK, Bolin K, et al. High genetic risk score is associated with early disease onset, damage accrual and decreased survival in systemic lupus erythematosus. Ann Rheum Dis. (2020) 79:363–9. 10.1136/annrheumdis-2019-216227 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Brown MA, Aletaha D. Genetic risk scores in inflammatory arthritis: a new era? Nat Rev Rheumatol. (2020) 16:545–6. 10.1038/s41584-020-0473-6 [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Knevel R, le Cessie S, Terao CC, Slowikowski K, Cui J, Huizinga TWJ, et al. Using genetics to prioritize diagnoses for rheumatology outpatients with inflammatory arthritis. Sci Transl Med. (2020) 12. 10.1126/scitranslmed.aay1548 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Haynes WA, Haddon DJ, Diep VK, Khatri A, Bongen E, Yiu G, et al. Integrated, multicohort analysis reveals unified signature of systemic lupus erythematosus. JCI Insight. (2020) 5. 10.1172/jci.insight.122312 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Mikdashi J, Nived O. Measuring disease activity in adults with systemic lupus erythematosus: the challenges of administrative burden and responsiveness to patient concerns in clinical research. Arthritis Res Ther. (2015) 17:183. 10.1186/s13075-015-0702-6 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Steiman AJ, Gladman DD, Ibañez D, Urowitz MB. Prolonged serologically active clinically quiescent systemic lupus erythematosus: frequency and outcome. J Rheumatol. (2010) 37:1822–7. 10.3899/jrheum.100007 [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Kegerreis B, Catalina MD, Bachali P, Geraci NS, Labonte AC, Zeng C, et al. Machine learning approaches to predict lupus disease activity from gene expression data. Sci Rep. (2019) 9:9617. 10.1038/s41598-019-45989-0 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 42. Stanley S, Vanarsa K, Soliman S, Habazi D, Pedroza C, Gidley G, et al. Comprehensive aptamer-based screening identifies a spectrum of urinary biomarkers of lupus nephritis across ethnicities. Nat Commun. (2020) 11:2197. 10.1038/s41467-020-16944-9 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA. (2003) 100:2610–5. 10.1073/pnas.0337679100 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Banchereau R, Hong S, Cantarel B, Baldwin N, Baisch J, Edens M, et al. Personalized immunomonitoring uncovers molecular networks that stratify lupus patients. Cell. (2016) 165:1548–50. 10.1016/j.cell.2016.05.057 [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Nehar-Belaid D, Hong S, Marches R, Chen G, Bolisetty M, Baisch J, et al. Mapping systemic lupus erythematosus heterogeneity at the single-cell level. Nat Immunol. (2020) 21:1094–106. 10.1038/s41590-020-0743-0 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Catalina MD, Bachali P, Geraci NS, Grammer AC, Lipsky PE. Gene expression analysis delineates the potential roles of multiple interferons in systemic lupus erythematosus. Commun Biol. (2019) 2:140. 10.1038/s42003-019-0382-x [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Eloranta ML, Rönnblom L. Cause and consequences of the activated type I interferon system in SLE. J Mol Med (Berl). (2016) 94:1103–10. 10.1007/s00109-016-1421-4 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Landolt-Marticorena C, Bonventi G, Lubovich A, Ferguson C, Unnithan T, Su J, et al. Lack of association between the interferon-alpha signature and longitudinal changes in disease activity in systemic lupus erythematosus. Ann Rheum Dis. (2009) 68:1440–6. 10.1136/ard.2008.093146 [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Petri M, Fu W, Ranger A, Allaire N, Cullen P, Magder LS, et al. Association between changes in gene signatures expression and disease activity among patients with systemic lupus erythematosus. BMC Med Genomics. (2019) 12:4. 10.1186/s12920-018-0468-1 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Imgenberg-Kreuz J, Carlsson Almlöf J, Leonard D, Alexsson A, Nordmark G, Eloranta ML, et al. DNA methylation mapping identifies gene regulatory effects in patients with systemic lupus erythematosus. Ann Rheum Dis. (2018) 77:736–43. 10.1136/annrheumdis-2017-212379 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Teruel M, Sawalha AH. Epigenetic variability in systemic lupus erythematosus: what we learned from genome-wide DNA methylation studies. Curr Rheumatol Rep. (2017) 19:32. 10.1007/s11926-017-0657-5 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Coit P, Dozmorov MG, Merrill JT, McCune WJ, Maksimowicz-McKinnon K, Wren JD, et al. Epigenetic reprogramming in naive CD4+ t cells favoring T cell activation and non-Th1 effector T cell immune response as an early event in lupus flares. Arthritis Rheumatol. (2016) 68:2200–9. 10.1002/art.39720 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Zhao M, Zhou Y, Zhu B, Wan M, Jiang T, Tan Q, et al. IFI44L promoter methylation as a blood biomarker for systemic lupus erythematosus. Ann Rheum Dis. (2016) 75:1998–2006. 10.1136/annrheumdis-2015-208410 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Coit P, Ortiz-Fernandez L, Lewis EE, McCune WJ, Maksimowicz-McKinnon K, Sawalha AH. A longitudinal and transancestral analysis of DNA methylation patterns and disease activity in lupus patients. JCI Insight. (2020) 5. 10.1172/jci.insight.143654 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Pan W, Zhu S, Yuan M, Cui H, Wang L, Luo X, et al. MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. J Immunol. (2010) 184:6773–81. 10.4049/jimmunol.0904060 [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Tang Y, Luo X, Cui H, Ni X, Yuan M, Guo Y, et al. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. (2009) 60:1065–75. 10.1002/art.24436 [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Hanly JG, O'Keeffe AG, Su L, Urowitz MB, Romero-Diaz J, Gordon C, et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology. (2016) 55:252–62. 10.1093/rheumatology/kev311 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. (2018) 93:789–96. 10.1016/j.kint.2017.11.023 [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Der E, Suryawanshi H, Morozov P, Kustagi M, Goilav B, Ranabothu S, et al. Tubular cell and keratinocyte single-cell transcriptomics applied to lupus nephritis reveal type I IFN and fibrosis relevant pathways. Nat Immunol. (2019) 20:915–27. 10.1038/s41590-019-0386-1 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. Wither JE, Prokopec SD, Noamani B, Chang NH, Bonilla D, Touma Z, et al. Identification of a neutrophil-related gene expression signature that is enriched in adult systemic lupus erythematosus patients with active nephritis: clinical/pathologic associations and etiologic mechanisms. PLoS ONE. (2018) 13:e0196117. 10.1371/journal.pone.0196117 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Arazi A, Rao DA, Berthier CC, Davidson A, Liu Y, Hoover PJ, et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat Immunol. (2019) 20:902–14. 10.1038/s41590-019-0398-x [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. Rao DA, Arazi A, Wofsy D, Diamond B. Design and application of single-cell RNA sequencing to study kidney immune cells in lupus nephritis. Nat Rev Nephrol. (2020) 16:238–50. 10.1038/s41581-019-0232-6 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 63. Catalina MD, Owen KA, Labonte AC, Grammer AC, Lipsky PE. The pathogenesis of systemic lupus erythematosus: harnessing big data to understand the molecular basis of lupus. J Autoimmun. (2020) 110:102359. 10.1016/j.jaut.2019.102359 [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. Mok CC, Mohan C. Urinary biomarkers in lupus nephritis: are we there yet? Arthritis Rheumatol. (2020) 73:194–6. 10.1002/art.41508 [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Fava A, Buyon J, Mohan C, Zhang T, Belmont HM, Izmirly P, et al. Integrated urine proteomics and renal single-cell genomics identify an IFN-γ response gradient in lupus nephritis. JCI Insight. (2020) 5. 10.1172/jci.insight.138345 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Wilhelm TR, Magder LS, Petri M. Remission in systemic lupus erythematosus: durable remission is rare. Ann Rheum Dis. (2017) 76:547–53. 10.1136/annrheumdis-2016-209489 [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Franklyn K, Lau CS, Navarra SV, Louthrenoo W, Lateef A, Hamijoyo L, et al. Definition and initial validation of a Lupus Low Disease Activity State (LLDAS). Ann Rheum Dis. (2016) 75:1615–21. 10.1136/annrheumdis-2015-207726 [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Zen M, Iaccarino L, Gatto M, Saccon F, Larosa M, Ghirardello A, et al. Lupus low disease activity state is associated with a decrease in damage progression in Caucasian patients with SLE, but overlaps with remission. Ann Rheum Dis. (2018) 77:104–10. 10.1136/annrheumdis-2017-211613 [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. Petri M, Magder LS. Comparison of remission and lupus low disease activity state in damage prevention in a United States Systemic Lupus Erythematosus Cohort. Arthritis Rheumatol. (2018) 70:1790–5. 10.1002/art.40571 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. Davidson JE, Fu Q, Rao S, Magder LS, Petri M. Quantifying the burden of steroid-related damage in SLE in the Hopkins Lupus Cohort. Lupus Sci Med. (2018) 5:e000237. 10.1136/lupus-2017-000237 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Abrahamowicz M, Esdaile JM, Ramsey-Goldman R, Simon LS, Strand V, Lipsky PE. Development and validation of a novel evidence-based lupus multivariable outcome score for clinical trials. Arthritis Rheumatol. (2018) 70:1450–8. 10.1002/art.40522 [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Furie RA, Petri MA, Wallace DJ, Ginzler EM, Merrill JT, Stohl W, et al. Novel evidence-based systemic lupus erythematosus responder index. Arthritis Rheum. (2009) 61:1143–51. 10.1002/art.24698 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 73. Tsokos GC, Lo MS, Costa Reis P, Sullivan KE. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol. (2016) 12:716–30. 10.1038/nrrheum.2016.186 [ DOI ] [ PubMed ] [ Google Scholar ]
- 74. Dall'Era M, Bruce IN, Gordon C, Manzi S, McCaffrey J, Lipsky PE. Current challenges in the development of new treatments for lupus. Ann Rheum Dis. (2019) 78:729–35. 10.1136/annrheumdis-2018-214530 [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Koelmeyer R, Nim HT, Nikpour M, Sun YB, Kao A, Guenther O, et al. High disease activity status suggests more severe disease and damage accrual in systemic lupus erythematosus. Lupus Sci Med. (2020) 7. 10.1136/lupus-2019-000372 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 76. Fawole OA, Dy SM, Wilson RF, Lau BD, Martinez KA, Apostol CC, et al. A systematic review of communication quality improvement interventions for patients with advanced and serious illness. J Gen Intern Med. (2013) 28:570–7. 10.1007/s11606-012-2204-4 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 77. Beusterien K, Bell JA, Grinspan J, Utset TO, Kan H, Narayanan S. Physician-patient interactions and outcomes in systemic lupus erythematosus (SLE): a conceptual model. Lupus. (2013) 22:1038–45. 10.1177/0961203313499958 [ DOI ] [ PubMed ] [ Google Scholar ]
- 78. Sloan M, Harwood R, Sutton S, D'Cruz D, Howard P, Wincup C, et al. Medically explained symptoms: a mixed methods study of diagnostic, symptom and support experiences of patients with lupus and related systemic autoimmune diseases. Rheumatol Adv Pract. (2020) 4:rkaa006. 10.1093/rap/rkaa006 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 79. Barbacki A, Petri M, Aviña-Zubieta A, Alarcón GS, Bernatsky S. Fatigue measurements in systemic lupus erythematosus. J Rheumatol. (2019) 46:1470–7. 10.3899/jrheum.180831 [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Tench CM, McCurdie I, White PD, D'Cruz DP. The prevalence and associations of fatigue in systemic lupus erythematosus. Rheumatology. (2000) 39:1249–54. 10.1093/rheumatology/39.11.1249 [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. (2005) 19:105–23. 10.2165/00023210-200519020-00002 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 82. Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, et al. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. (2009) 65:296–303. 10.1016/j.biopsych.2008.08.010 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 83. Schwarting A, Möckel T, Lütgendorf F, Triantafyllias K, Grella S, Boedecker S, et al. Fatigue in SLE: diagnostic and pathogenic impact of anti-N-methyl-D-aspartate receptor (NMDAR) autoantibodies. Ann Rheum Dis. (2019) 78:1226–34. 10.1136/annrheumdis-2019-215098 [ DOI ] [ PubMed ] [ Google Scholar ]
- 84. Strand V, Levy RA, Cervera R, Petri MA, Birch H, Freimuth WW, et al. Improvements in health-related quality of life with belimumab, a B-lymphocyte stimulator-specific inhibitor, in patients with autoantibody-positive systemic lupus erythematosus from the randomised controlled BLISS trials. Ann Rheum Dis. (2014) 73:838–44. 10.1136/annrheumdis-2012-202865 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 85. Rainey H, Bell K, Rus V, Wallace D, Dykas C, Mora M, et al. Delayed and immediate release prednisone decrease fatigue comparably in patients with systemic lupus erythematosus. ACR Convergence 2020. Arthritis Rheumatol. (2020). [ Google Scholar ]
- 86. Lever E, Alves MR, Isenberg DA. Towards precision medicine in systemic lupus erythematosus. Pharmgenomics Pers Med. (2020) 13:39–49. 10.2147/PGPM.S205079 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 87. Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res. (2012) 64:797–808. 10.1002/acr.21664 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. Andreoli L, Bertsias GK, Agmon-Levin N, Brown S, Cervera R, Costedoat-Chalumeau N, et al. EULAR recommendations for women's health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis. (2017) 76:476–85. 10.1136/annrheumdis-2016-209770 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 89. Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. (2019). 78:736–45. 10.1136/annrheumdis-2019-215089 [ DOI ] [ PubMed ] [ Google Scholar ]
- 90. Morand EF, Furie R, Tanaka Y, Bruce IN, Askanase AD, Richez C, et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med. (2020) 382:211–21. 10.1056/NEJMoa1912196 [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Wallace DJ, Kalunian K, Petri MA, Strand V, Houssiau FA, Pike M, et al. Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study. Ann Rheum Dis. (2014) 73:183–90. 10.1136/annrheumdis-2012-202760 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 92. Ward MM, Marx AS, Barry NN. Comparison of the validity and sensitivity to change of 5 activity indices in systemic lupus erythematosus. J Rheumatol. (2000) 27:664–70. [ PubMed ] [ Google Scholar ]
- 93. Pitzalis C, Choy EHS, Buch MH. Transforming clinical trials in rheumatology: towards patient-centric precision medicine. Nat Rev Rheumatol. (2020) 16:590–9. 10.1038/s41584-020-0491-4 [ DOI ] [ PubMed ] [ Google Scholar ]
- 94. Felten R, Scher F, Sagez F, Chasset F, Arnaud L. Spotlight on anifrolumab and its potential for the treatment of moderate-to-severe systemic lupus erythematosus: evidence to date. Drug Des Devel Ther. (2019) 13:1535–43. 10.2147/DDDT.S170969 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 95. Merrill JT, Wallace DJ, Wax S, Kao A, Fraser PA, Chang P, et al. Efficacy and safety of atacicept in patients with systemic lupus erythematosus: results of a twenty-four-week, multicenter, randomized, Double-blind, placebo-controlled, parallel-arm, phase IIb study. Arthritis Rheumatol. (2018) 70:266–76. 10.1002/art.40360 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 96. Furie R, Khamashta M, Merrill JT, Werth VP, Kalunian K, Brohawn P, et al. Anifrolumab, an anti-interferon-α receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol. (2017) 69:376–86. 10.1002/art.39962 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 97. Merrill J. Identifying an SLE patient cluster with greater treatment effect: immune cell deconvolution of gene expression in two atacicept phase II studies Arthritis Rheumatol . (2020). [ Google Scholar ]
- 98. Rydén-Aulin M, Boumpas D, Bultink I, Callejas Rubio JL, Caminal-Montero L, Castro A, et al. Off-label use of rituximab for systemic lupus erythematosus in Europe. Lupus Sci Med. (2016) 3:e000163. 10.1136/lupus-2016-000163 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 99. Tony HP, Burmester G, Schulze-Koops H, Grunke M, Henes J, Kötter I, et al. Safety and clinical outcomes of rituximab therapy in patients with different autoimmune diseases: experience from a national registry (GRAID). Arthritis Res Ther. (2011) 13:R75. 10.1186/ar3337 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 100. Owen KA, Price A, Ainsworth H, Aidukaitis BN, Bachali P, Catalina MD, et al. Analysis of trans-ancestral SLE risk loci identifies unique biologic networks and drug targets in African and European Ancestries. Am J Hum Genet. (2020) 107:864–81. 10.1016/j.ajhg.2020.09.007 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 101. Dudley JT, Sirota M, Shenoy M, Pai RK, Roedder S, Chiang AP, et al. Computational repositioning of the anticonvulsant topiramate for inflammatory bowel disease. Sci Transl Med. (2011) 3:96ra76. 10.1126/scitranslmed.3002648 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 102. Kingsmore KM, Grammer AC, Lipsky PE. Drug repurposing to improve treatment of rheumatic autoimmune inflammatory diseases. Nat Rev Rheumatol. (2020) 16:32–52. 10.1038/s41584-019-0337-0 [ DOI ] [ PubMed ] [ Google Scholar ]
- 103. Grammer AC, Ryals MM, Heuer SE, Robl RD, Madamanchi S, Davis LS, et al. Drug repositioning in SLE: crowd-sourcing, literature-mining and Big Data analysis. Lupus. (2016) 25:1150–70. 10.1177/0961203316657437 [ DOI ] [ PubMed ] [ Google Scholar ]
- 104. Toro-Domínguez D, Lopez-Domínguez R, García Moreno A, Villatoro-García JA, Martorell-Marugán J, Goldman D, et al. Differential treatments based on drug-induced gene expression signatures and longitudinal systemic lupus erythematosus stratification. Sci Rep. (2019) 9:15502. 10.1038/s41598-019-51616-9 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
- View on publisher site
- PDF (531.3 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Lupus Science & Medicine®
is a global, peer-reviewed, open access lupus journal from LFA, publishing papers on all aspects of lupus and related diseases.
Impact factor: 3.7 Citescore: 5.3 All metrics >>
Lupus Science & Medicine® is a global, peer reviewed, open access online journal that provides a central point for publication of basic, clinical, translational, and epidemiological studies of all aspects of lupus and related diseases.
An official journal of the Lupus Foundation of America (LFA), which is dedicated to advancing the science and medicine of lupus while offering support to patients and their caregivers.
Follow the journal via X and Facebook .
Editors-in-Chief: Professor Jill Buyon, New York University, USA and Professor Ronald van Vollenhoven, Amsterdam University Medical Centers, The Netherlands Editorial team

Publish in LSM
Lupus Science & Medicine considers submissions of a range of article types, including original research, brief communications, study protocols and meeting reports.
Information is also provided on editorial policies and open access .
This is an open access journal that levies an Article Publishing Charge (APC). Discounts and waivers are available; details are provided in the Author Information section.
All manuscripts should be submitted online.
Published articles are discoverable via PubMed, Web of Science, Scopus and Google Scholar.
Video: Benefits of publishing in Lupus Science & Medicine
Latest Articles
Reproductive health and APS :
12 December 2024
Epidemiology and outcomes :
11 December 2024
9 December 2024
Lupus nephritis :
28 November 2024
27 November 2024
Lupus Academy 2024
Lupus Science & Medicine was delighted to be the official journal of the 13th annual meeting of the Lupus Academy 2024. The meeting took place 6-8 September in Amsterdam, The Netherlands.
The abstracts from the conference were published as a supplement to Lupus Science & Medicine .

LSM Podcasts
Featured video, lupus science & medicine.
The official journal of the Lupus Foundation of America and the premier open-access journal dedicated to lupus research.

Most Read Articles
22 May 2024
15 February 2024
19 January 2024
Clinical trials and drug discovery :
7 March 2024
Childhood lupus :
Related Journals

Annals of the Rheumatic Diseases


Lupus Science & Medicine®

Lupus Science & Medicine ® is a peer-reviewed, open access, online journal that provides a central point for publication of basic, clinical, translational, and epidemiological studies of all aspects of lupus and related diseases.
It is the first lupus-specific open access journal in the world and was developed in response to the need for a barrier-free forum for publication of groundbreaking lupus studies. Lupus Science & Medicine ® (LS&M) is owned by the Lupus Foundation of America and published by BMJ, a respected global provider of evidence-based medical knowledge.
In 2023, LS&M received an Impact Factor (IF) of 3.9. This is the highest current ranking IF for any lupus-specific journal and is closely ranked among the most prominent journals in rheumatology. The LS&M Scopus CiteScore is now 5.
Below, LS&M Co-Editor-in-Chief, Dr. Jill Buyon, discusses what makes this journal so special for lupus researchers and clinicians.

Research on lupus will be considered from fields including, but not limited to, rheumatology, dermatology, nephrology, immunology, pediatrics, cardiology, hepatology, pulmonology, obstetrics and gynecology, and psychiatry. Submissions from groups of investigators engaged in international collaborations are especially encouraged.
New articles are added year-round so the online content always includes the latest developments in lupus research and treatment.
Articles cover the latest developments from basic, clinical, translational, and epidemiological studies of all aspects of lupus and related diseases.
Want to know about the hottest topics in lupus research? Check out the list of 'most read articles' on Lupus Science & Medicine®.
Be the first to know. Sign up to receive an eTOC Alerts when a new article is published on Lupus Science & Medicine®.
Authors discuss their research findings and provide additional insight into their conclusions.
Yearly Online Visits
Journal Article Views in 2023
Mentions of Journal Content on Social Media & in News Outlets
Lupus Science & Medicine ® is edited by Jill P. Buyon, Division Director of Rheumatology, Professor of Medicine at New York University School of Medicine; and Ronald F. van Vollenhoven, Professor and Chief, Unit for Clinical Therapy Research, Inflammatory Diseases (ClinTRID), The Karolinska Institute, Stockholm, Sweden. The journal has also assembled a world-class team of associate editors and an editorial board to maintain the journal's high standards, high visibility, and credibility.
In January of 2022, Dr. Joan Merrill joined the Lupus Science and Medicine board as an Associate Editor. Dr. Merrill is the Chief Advisor for Clinical Development for the Lupus Foundation of America (LFA) and is a major leader in lupus trial design, innovating new ways to tackle the heterogeneity inherent in this complex disease. In addition to her own studies, Dr. Merrill also works with the LFA to train investigators for trials worldwide.
We look forward to working with Dr. Merrill, and thank her in advance for the valued contributions she will bring to the journal.
Lupus Science & Medicine ® complements the Lupus Foundation of America’s national research program , which pursues an aggressive agenda to tackle the biggest challenges in lupus and find answers to the most important questions.
There are multiple chapters near you. Select your preferred chapter.
Supporting lupus patients and advocates in Arizona.
Serving the District of Columbia, Maryland, and Northern Virginia.
Serving Kansas, Missouri, and Central & Southern Illinois
Serving north, central and west Texas, including Dallas/Fort Worth, San Antonio, Austin, El Paso, Lubbock, and surrounding areas
Serving Northern Illinois, Indiana, Iowa, Michigan, and Minnesota
Serving Connecticut, Massachusetts, Maine, New Hampshire, northern and central New Jersey, New York, Rhode Island, and Vermont
Serving Pennsylvania, Delaware and Southern New Jersey
Serving Houston, Beaumont, Corpus Christi, Harlingen and surrounding areas in Texas
There are multiple walks near you.
This website uses cookies to ensure you get the best experience. Learn more
The 100 top-cited studies in systemic lupus erythematosus: A bibliometric analysis
Affiliations.
- 1 Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
- 2 Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China.
- 3 Tianjin Institutes of Health Science, Tianjin, China.
- 4 State Key Laboratory of Experimental Hematology, National Clinical Research Center for Blood Diseases, Haihe Laboratory of Cell Ecosystem, Tianjin Key Laboratory of Gene Therapy for Blood Diseases, CAMS Key Laboratory of Gene Therapy for Blood Diseases, Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin, China.
- 5 West China School of Medicine, Sichuan University, Chengdu, China.
- 6 School of Clinical Medicine, Southwest Medical University, Luzhou, China.
- 7 Key Laboratory of Rheumatology & Clinical Immunology, Ministry of Education, Beijing, China.
- PMID: 39149877
- PMCID: PMC11328883
- DOI: 10.1080/21645515.2024.2387461
Systemic lupus erythematosus (SLE) is an autoimmune inflammatory tissue disease. In view of the explosive growth in research on SLE, bibliometrics was performed to evaluate the 100 top-cited papers in this realm. We performed the search with terms "systemic lupus erythematosus" the Web of Science Core Collection database on May 3, 2023. Relevant literatures were screened. Data were extracted and analyzed by SPSS. The citations of 100 top-cited SLE studies spanned from 472 to 13,557. Most studies (60 out of 100) were conducted in the United States. Total citation times were positively associated with ACY, which was negatively correlated with the length of time since publication. Approximately half of the studies focused on the underlying mechanisms of SLE. New biologic therapies garnered attention and development. Our findings provide valuable insights into the developments in crucial areas of SLE and shed contributions to future studies.
Keywords: Systemic lupus erythematosus; bibliometric analysis; bibliometrics; citations; literature review; research hotspots.
Publication types
- Bibliometrics*
- Lupus Erythematosus, Systemic*
Grants and funding

IMAGES
VIDEO
COMMENTS
Submit Paper. The only fully peer reviewed international journal devoted exclusively to lupus (and related disease) research. Lupus includes the most promising new clinical and laboratory-based studies from leading specialists in all lupus-related disciplines. Invaluable reading, with extended coverage, lupus-related disciplines include ...
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease presenting highly heterogeneous clinical manifestations and multi-systemic involvement. ... 1 Center of Research of Immunopathology and Rare Diseases, Coordinating Center of Piemonte and Valle d'Aosta Network for Rare Diseases, Department of Clinical and Biological Sciences ...
Systemic lupus erythematosus (SLE) is a complex autoimmune disease with multisystem involvement. It is multifactorial and involves epigenetic, genetic, ecological, and environmental factors. ... Current research is on its way, and further randomized control trials are needed in order to develop targeted therapies. Management of chronic diseases ...
Systemic lupus erythematosus is a potentially fatal autoimmune disease that is characterized by wide-spread inflammation and tissue damage that can affect any part of the body. Commonly affected ...
The vastly diverse nature of systemic lupus erythematosus (SLE) poses great challenges to clinicians and patients, as well as to research and drug development efforts. Precise management of lupus patients would be advanced by the ability to identify specific abnormalities operative in individual patients at the time of encounter with the clinician.
Systemic lupus erythematosus (SLE) is an autoimmune inflammatory tissue disease. In view of the explosive growth in research on SLE, bibliometrics was performed to evaluate the 100 top-cited papers in this realm. We performed the search with terms "systemic lupus erythematosus" the Web of Science Core Collection database on May 3, 2023.
Lupus Science & Medicine® is a global, peer reviewed, open access online journal that provides a central point for publication of basic, clinical, translational, and epidemiological studies of all aspects of lupus and related diseases. An official journal of the Lupus Foundation of America (LFA), which is dedicated to advancing the science and medicine of lupus while offering support to ...
Lupus Science & Medicine® is a peer-reviewed, open access, online journal that provides a central point for publication of basic, clinical, translational, and epidemiological studies of all aspects of lupus and related diseases.. It is the first lupus-specific open access journal in the world and was developed in response to the need for a barrier-free forum for publication of groundbreaking ...
Systemic lupus erythematosus (SLE) is an autoimmune inflammatory tissue disease. In view of the explosive growth in research on SLE, bibliometrics was performed to evaluate the 100 top-cited papers in this realm. We performed the search with terms "systemic lupus erythematosus" the Web of Science Core Collection database on May 3, 2023.
Systemic lupus erythematosus (SLE) is a genetically predisposed, female-predominant disease, characterized by multiple organ damage, that in its most severe forms can be life-threatening. The pathogenesis of SLE is complex and involves cells of both innate and adaptive immunity. ... Feature papers represent the most advanced research with ...