An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Dietary carbohydrate modifies the density of L cells in the chicken ileum
Md salahuddin, kohzy hiramatsu, iori nishimoto, kazumi kita.
- Author information
- Article notes
- Copyright and License information
Correspondence to: Hiramatsu, K.: [email protected]
Received 2021 Oct 26; Accepted 2021 Dec 20; Issue date 2022 Feb.
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (by-nc-nd) License. (CC-BY-NC-ND 4.0: https://creativecommons.org/licenses/by-nc-nd/4.0/ )
Glucagon-like peptides (GLPs) are secreted from intestinal L cells and stimulate various physiological functions in the gastrointestinal tract. The secretion of GLPs is influenced by macronutrient ingestion. This study aims to clarify the effects of dietary carbohydrate (CHO) on L cells in the chicken ileum. Six-week-old, male White Leghorn chickens were divided into three groups: control, low-CHO and CHO-free, with five chickens in each group. Paraffin sections were made from the proximal and distal ileum of each animal and subjected to immunohistochemistry for GLP-1 and GLP-2 peptides and in situ hybridization for proglucagon (PG) mRNA. A significant reduction of GLP-1- and GLP-2-immunoreactive cells was observed in the two experimental groups compared with that in the control. A reduction of cells expressing PG mRNA was observed in the proximal and distal ileum of the CHO-free group compared with that in the control. The ratio of GLP-1-immunoreactive cells showing Ki-67 immunoreactivity was significantly lower in the distal ileum of the CHO-free group than that in the control group. These data suggest that dietary CHO is an effective stimulator for modifying L cell density in the chicken ileum.
Keywords: carbohydrate, chicken, glucagon-like peptides, Ki-67, proglucagon mRNA
Glucagon-like peptide (GLP) is one of the major intestinal hormones and has two subtypes, GLP-1 and GLP-2. GLP-1 is a 36-amino acid peptide released from intestinal L cells in response to ingested nutrients [ 1 ]. In the gut, this peptide hormone performs a variety of physiological and biological roles. Some of these functions include enhancing glucose-dependent insulin release, inhibiting glucagon secretion, increasing pancreatic β cell growth [ 10 , 27 ], decreasing food intake [ 1 ], decelerating gastric emptying [ 27 ], inhibiting intestinal motility [ 36 ], and stimulating intestinal growth [ 21 ].
GLP-2 is a 33-amino acid intestinotrophic peptide [ 35 ] and has a range of effects on the gastrointestinal (GI) tract. This hormone promotes intestinal development by increasing epithelial proliferation and decreasing apoptosis [ 13 ]. GLP-2 also suppresses gastric acid secretion, reduces gastric motility, and regulates food intake [ 3 , 6 , 38 ].
GLP-1 and GLP-2 are cleaved from their common precursor protein, proglucagon (PG), in intestinal L cells by unique post-translational proteolytic cleavage and other enzymatic changes [ 4 , 9 ]. L cells are widely found in the small intestine and colon of mammals [ 23 ]. In chickens, L cells are located throughout the entire jejunum and ileum [ 18 , 19 ] and L cell apical surface is covered with microvilli [ 28 ]. These microvilli contain several receptors and channels that receive chemical signals from the gut lumen, making them open-type enteroendocrine cells (EECs) [ 15 ].
The synthesis of GLPs positively correlates with the density of intestinal L cells [ 5 ]. Consequently, an increase in L cell density may enhance the physiological and biological activities of GLPs in the GI tract. In fact, our previous studies in chickens demonstrated that dietary protein and the supplement of amino acids increased the density of GLP-1-immunoreactive cells in the chicken ileum [ 24 , 29 ]. However, relatively little data exist on the impact of dietary carbohydrate (CHO) levels on the density of L cells in the chicken ileum.
We recently revealed that dietary CHO positively affected the proliferation of epithelial cells in the chicken ileum [ 34 ]. In the present study, we aimed to evaluate the effect of dietary CHO on the density and proliferation of L cells in the chicken ileum using immunohistochemistry and in situ hybridization.
MATERIALS AND METHODS
The animal experiment protocol was reviewed by the Committee for Animal Experiments and approved by the president of Shinshu University (Approval Number 300090). Chickens were kept in the facility of the experiments for avian species of the Faculty of Agriculture, Shinshu University.
Animals and feeding management
Six-week-old, male White Leghorn chickens (n=15) were used in this study. They were assigned into the control, low-CHO, and CHO-free groups based on the average body weight. Each group consisted of five birds. Initially, all chickens received the control diet in separated cages for 3 days to acclimate them to the experimental circumstances. Subsequently, each experimental diet was supplied to the corresponding group for 7 days. Experimental diets for low-CHO and CHO-free groups contained 12.5% and 0% CHO of that of the control group which contains 491.4 g CHO/kg diet, respectively. Metabolizable energy (ME) of the two experimental diets was sustained at the same level as the control diet (ME=2,850 kcal/kg) by adding corn oil and cellulose. Therefore, the energy content of the diet satisfied the ME requirement specified by the Japanese Feeding Standard for Poultry [ 26 ]. Chickens could access feed and water freely during the experimental period. The daily feed intake and body weight of each chicken were measured at the same time during the experimental period.
Sample collection
All chickens were sacrificed through decapitation under anesthesia with sodium pentobarbital injection on the last day of the experimental period. Tissue samples approximately 2 cm long were taken from the proximal and distal parts of the ileum of each bird. After washing in 0.75% NaCl solution, tissue samples were fixed in Bouin’s solution for 24 hr at 4°C and then embedded in paraffin wax, according to the standard method. Paraffin sections cut at 5 μm thickness from each tissue sample were used in the following procedures.
Immunohistochemistry
The streptavidin-biotin method [ 14 ] was used to identify both GLP-1- and GLP-2-immunoreactive cells [ 16 ]. For the detection of GLP-1 immunoreactivity, sections treated with normal goat serum were incubated with rabbit antiserum against synthetic GLP-1 (1–19) (Affiniti Research Products, Devon, UK, No. GA1176, diluted to 1:2,000) for 24 hr at room temperature. For the detection of GLP-2 immunoreactivity, sections were first treated with a 0.5% antigen retrieval agent (Immunosaver ® , Nisshin EM, Tokyo, Japan) at 98°C for 45 min [ 25 ]. They were then incubated with rabbit antiserum against human (Arg 34 )-GLP-2 (1:2,000, H-028-14, Phenix Pharmaceuticals, Burlingame, CA, USA) as a primary antibody for 24 hr following the treatment with 10% normal goat serum.
After several washes with phosphate buffered saline (PBS), all sections were incubated with biotin-labeled goat antiserum against rabbit immunoglobulin G (IgG, AP132B, Millipore, Temecula, CA, USA, 1:300) and streptavidin-polyHRP20 (SP20C, Stereospecific Detection Technologies, Baesweiler, Germany, 1:300) as the secondary antibody and label for immunocomplex, respectively. Immunocomplex was visualized through incubation with a 3,3′-diaminobenzidine-hydrogen peroxide solution. Preparations were counterstained with Mayer’s hematoxylin and observed under a light microscope. All incubations were performed in a moisture chamber at room temperature.
Morphometry
The occurrence of GLP-1- and GLP-2-immunoreactive cells was assessed in the following manner [ 19 ]. First, immunoreactive cells for GLP-1 and GLP-2 antisera with a clearly visible nucleus were counted on a microphotograph taken at 5× magnification. Second, the mucosal area was measured and the frequency of immunoreactive cells (cells/mm 2 ± SD) was calculated. A computerized image analysis system (KS-400, ZEISS, Göttingen, Germany) was used for this quantification. Twenty areas were randomly selected from each ileal part of each chicken. In total, 100 areas of each ileal part were assessed from the five chickens in each group.
In situ hybridization and double immunofluorescence method
Several sets of mirror sections cut at 5 μm thickness were utilized in the following procedures. An oligonucleotide probe labeled with digoxigenin (DIG) was commercially synthesized (BEX, Tokyo, Japan) according to the chicken PG sequence shown by Richards and McMurtry [ 32 ]. Sequences of PG antisense and sense probes were 5′-GCTGTAGTCACTGGTGAATGTGCCTTGTGAATGACGCTTTA-3′ and 5′-TAAAGCGTCATTCACAAGGCACATTCACCAGTGACTACAGC-3′, respectively. The signal of PG mRNA was detected using a commercial in situ hybridization kit (IsHyb In Situ Hybridization Kit; Biochain Institute, Newark, CA, USA) on a pair of mirror section [ 37 ]. The sections were then treated with anti-DIG serum diluted with alkaline phosphatase solution (1:500), and the conjugated probe was visualized using the mixture solution of nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate. The sense probe was utilized as a negative control for PG mRNA.
Double immunofluorescence for GLP-1 and GLP-2 was conducted on complementary sections of mirror sections stained for in situ hybridization mentioned above. Sections treated with antigen retrieval agent (Immunosaver ® ) were incubated with 10% normal goat serum. They were treated with a mouse monoclonal antibody against synthetic human GLP-1 (aa 7-36 amide) (A6104.1, Immunodiagnostik, Bensheim, Germany, 1:2,000) for 24 hr. Samples were subsequently treated with a rabbit antiserum against human (Arg 34 )-GLP-2 serum (1:500, H-028-14, Phenix Pharmaceuticals) for 24 hr. After washing with PBS, sections were incubated with a cocktail of DyLight 488-conjugated goat anti-mouse IgG (611-741-127; Rockland Immunochemicals, Gilbertsville, PA, USA, diluted to 1:300) and DyLight 549-conjugated goat anti-rabbit IgG (611-700-127; Rockland Immunochemicals, diluted to 1:300) for 3 hr at room temperature. Finally, sections were covered with coverslips in an aqueous mounting medium and photographed using a fluorescence microscope (AxioImagerA1, Zeiss).
Immunohistochemistry for Ki-67 to determine L cells proliferation
Double immunofluorescence using an antiserum against Ki-67 was conducted to evaluate the influence of dietary CHO on L cell proliferation. Ki-67 is expressed as a nonhistone nucleoprotein during all stages of the cell cycle [ 22 ], indicating that it is valuable as a marker of cell proliferation. Paraffin sections were treated with 10% HistoVT one (Nacalai tesque, Kyoto, Japan) as an antigen retrieval agent for 20 min at 90°C. After several PBS washes, sections were treated with 2.5% normal donkey serum, and incubated with mouse monoclonal antibody against Ki-67 (MAB4190, Sigma-Aldrich, St. Louis, MO, USA, 1:500) for 24 hr. Following PBS washing, sections were treated with 10% normal goat serum and incubated with rabbit antiserum against synthetic GLP-1 (1–19) conjugated to bovine serum. Immunocomplexes were revealed using a cocktail of DyLight 549-conjugated donkey anti-mouse IgG serum and FITC-conjugated goat anti-rabbit IgG serum for 3 hr. All incubations took place in a moisture chamber at room temperature.
Statistical analysis
Frequencies of GLP-1- and GLP-2-immunoreactive cells were given as mean ± standard error of the mean. One-way analysis of variance was used. Additionally, Tukey’s test was used to assess any significant differences in the mean among groups. The significance level for the data was set at P <0.05. All statistical analyses were conducted using statistical analysis software (SAS, Inst. Inc., Cary, NC, USA).
Effects of dietary CHO on GLP-1- and GLP-2-immunoreactive cells
Many endocrine cells immunoreactive for GLP-1 antiserum were found throughout the ileum. GLP-1-immunoreactive cells were scattered in the epithelium of middle to bottom parts of villi and crypts ( Fig. 1Aa, 1Ad , arrows) in the proximal and distal ileum. There were no discernible variations in the distribution of GLP-1-immunoreactive cells among the control and the two experimental groups. In the control group, GLP-1-immunoreactive cells had a spindle-like or pyramidal shape with a long cytoplasmic process reaching the intestinal lumen in the villus epithelium ( Fig. 1Ab , arrows). These cells demonstrated a comma-like shape in crypts ( Fig. 1Ac , arrow). GLP-1-immunoreactive cells in the two experimental groups were found in an oval or round shape in both the villus epithelium ( Fig. 1Ae , arrows) and crypts ( Fig. 1Af ). Figure 1B and 1C show the frequencies of GLP-1-immunoreactive cells in the proximal and distal ileum, respectively. The frequency of GLP-1-immunoreactive cells was significantly reduced with the decrease of dietary CHO level in both ileal parts, with the lowest measured in the CHO-free group.
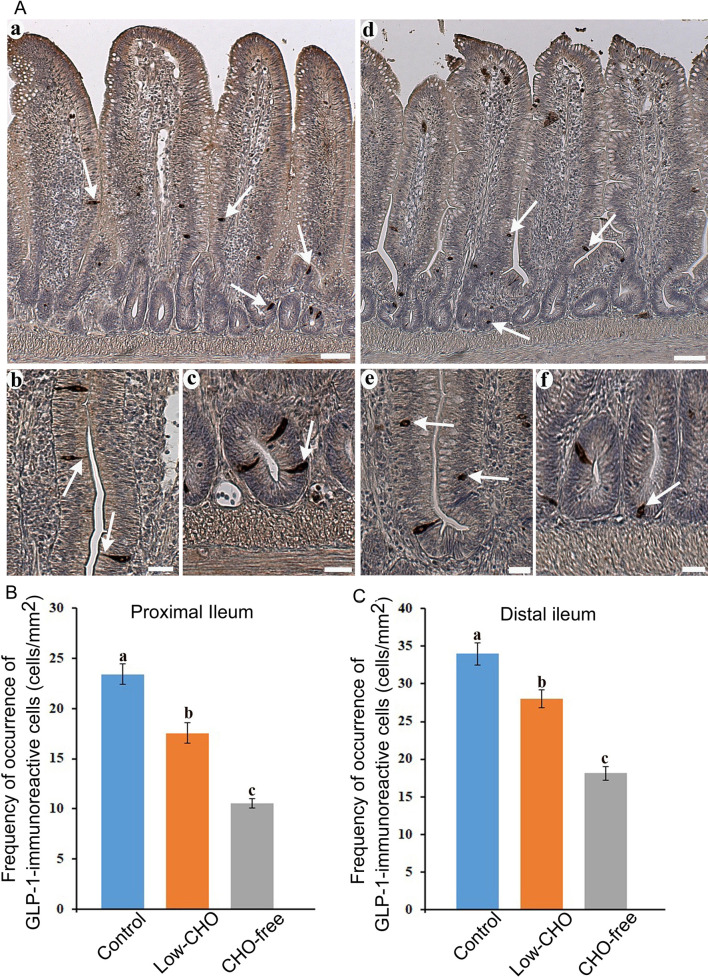
Glucagon-like peptide (GLP)-1-immunoreactive cells in the chicken ileum. A : Photomicrographs showing the distribution of GLP-1-immunoreactive cells (arrows) in the distal ileum from the control (a) and carbohydrate (CHO)-free (d) groups. Bars: 50 μm. High magnification views of GLP-1-immunoreactive cells in the villus epithelium (b, e) and crypts (c, f). A long cytoplasmic process of GLP-1-immunoreactive cells reaches the intestinal lumen. In the CHO-free group, GLP-1-immunoreactive cells in a round or oval shape are frequently observed. Bars: 20 μm. B : Frequency of occurrence of GLP-1-immunoreactive cells in the proximal ileum from the control, low-CHO, and CHO-free groups. There is a significant difference between different alphabets. P <0.05. Error bars: Standard error. a>b>c. C : Frequency of occurrence of GLP-1-immunoreactive cells in the distal ileum from the control, low-CHO, and CHO-free groups. There is a significant difference between different alphabets. P <0.05. Error bars: Standard error. a>b>c.
Many GLP-2-immunoreactive cells were also observed in the whole ileum. GLP-2-immunoreactive cells were detected in the epithelium of middle to bottom parts of the villi ( Fig. 2Aa , arrows) and crypts ( Fig. 2Ad , arrows). No apparent differences were observed in the distribution pattern of GLP-2-immunoreactive cells between the control and the experimental groups. Most of the cells in the control group exhibited a spindle-like shape with a long cytoplasmic process reaching the intestinal lumen in the villus epithelium ( Fig. 2Ab , arrow) and a comma-like shape in crypts ( Fig. 2Ac , arrows). In the two experimental groups, especially in the CHO-free group, GLP-2-immunoreactive cells in the villus epithelium ( Fig. 2Ae , arrows) and crypts ( Fig. 2Af , arrows) were more oval or round than those in the control group. Figure 2B and 2C show the frequencies of GLP-2-immunoreactive cells in the proximal and distal ileum, respectively. There were significant differences in the frequency of GLP-2-immunoreactive cells between the control and the experimental groups in both the proximal and distal ileum. GLP-2-immunoreactive cells were significantly reduced with the decrease of dietary CHO level in both ileal parts, and were lowest in the CHO-free group.
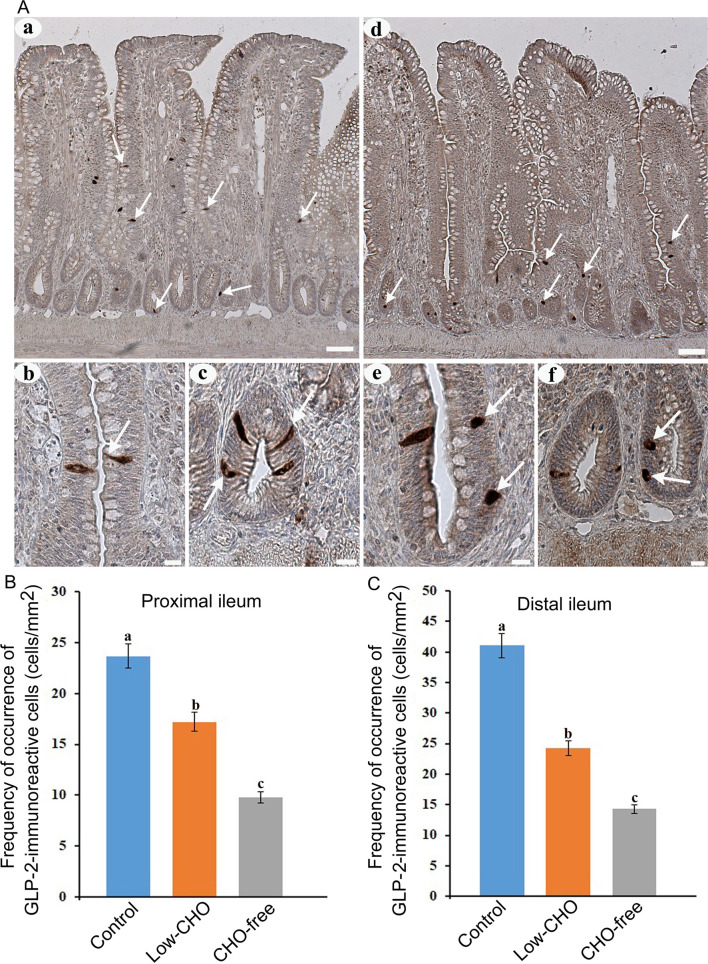
Glucagon-like peptide (GLP)-2-immunoreactive cells in the chicken ileum. A : Photomicrographs showing the distribution of GLP-2-immunoreactive cells (arrows) in the distal ileum from the control (a) and carbohydrate (CHO)-free (e) groups. Bars: 50 μm. High magnification views of GLP-2-immunoreactive cells in villus epithelium (b, e) and crypts (c, f). A long cytoplasmic process of GLP-2-immunoreactive cells reaches the intestinal lumen (arrow in b). In the CHO-free group, GLP-2-immunoreactive cells in a round or oval shape are frequently observed (arrows in f). Bars: 20 μm. B : Frequency of occurrence of GLP-2-immunoreactive cells in the proximal ileum from the control, low-CHO, and CHO-free groups. There is a significant difference between different alphabets. P <0.05. Error bars: Standard error. a>b>c. C : Frequency of occurrence of GLP-2-immunoreactive cells in the distal ileum from the control, low-CHO, and CHO-free groups. There is a significant difference between different alphabets. P <0.05. Error bars: Standard error. a>b>c.
Effects of dietary CHO on PG mRNA signals in the ileum
Cells expressing PG mRNA signal were identified in the proximal and distal ileum in the control and both experimental groups ( Fig. 3 , top panels). These were mainly located in the epithelium of the bottom part of villi and crypts. There were no distinguishable differences in the distribution pattern of cells expressing PG mRNA signal between the control and the experimental groups for either part of the ileum. Sense probe control did not produce any discernible PG mRNA signal ( Fig. 3 , bottom panels).
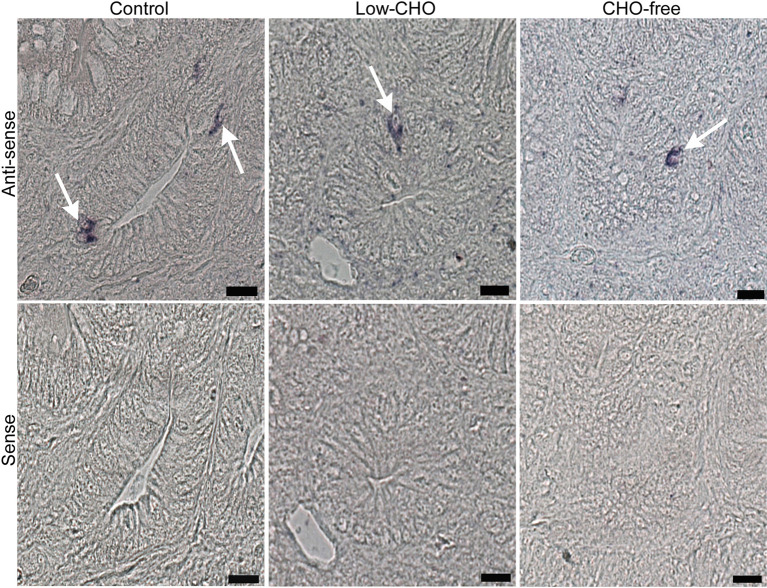
Photomicrographs indicating cells expressing proglucagon (PG) mRNA signal in the chicken distal ileum from the control, low-carbohydrate (CHO), and CHO-free groups. Antisense probe shows cells expressing PG mRNA signal (arrows in top panels) mainly in crypts of all groups. However, the sense probe shows no cells showing PG mRNA signal (bottom panels). Bars: 20 μm.
However, apparent differences were observed in the relative frequency of cells expressing PG mRNA signal among the three groups ( Table 1 ). The CHO-free group showed a lower frequency of cells expressing PG mRNA signal in the proximal ileum compared with that in the other two groups. There were no discernible differences between the control and the low-CHO groups. The relative frequency of cells expressing PG mRNA signal was reduced with the decrease of dietary CHO level in both ileal parts, with the lowest in the CHO-free group.
Table 1. Relative frequency of cells expressing proglucagon mRNA signal in the proximal and distal ileum of chickens.
+ +=moderate; +=few; ± =rarely; CHO=carbohydrate.
Most cells expressing PG mRNA signal exhibited immunoreactivity for both GLP-1 and GLP-2 in the proximal and distal ileum ( Fig. 4a–c , arrows). However, there was no clear difference in the ratio of cells expressing PG mRNA signal and GLP immunoreactivity among the three groups. Cells expressing only PG mRNA signal were rarely observed in the control and two experimental groups ( Fig. 4d–f , arrows).
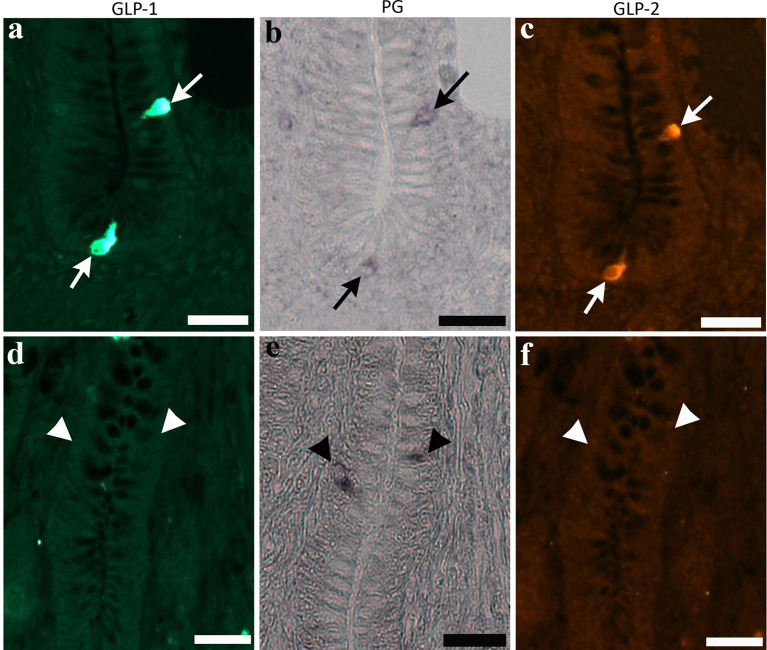
Photomicrographs indicate cells expressing immunoreactivity for glucagon-like peptide (GLP)-1 ( a ) and GLP-2 ( c ), and signal of proglucagon (PG) mRNA ( b ) in the bottom part of villi of the chicken distal ileum from the control group (arrows in top panels). Same cell types are also observed in the two experimental groups. Cells expressing only PG mRNA signal (arrowheads in bottom panels, e ), but not immunoreactivity for GLP-1 ( d ) and GLP-2 ( f ), are rarely observed in crypts. Bars: 20 μm.
Effects of dietary CHO on ileal L cell proliferation
Double immunofluorescence revealed the coexpression of Ki-67 and GLP-1 immunoreactivity in the same cell ( Fig. 5A , arrows in top panels). The ratio of GLP-1-immunoreactive cells also showing Ki-67 immunoreactivity was similar in the proximal ileum among the three groups ( Fig. 5B ). However, this was reduced with the decrease of dietary CHO level in the distal ileum. A significant difference was revealed between the control and the CHO-free groups ( Fig. 5C ). GLP-1-immunoreactive cells without Ki-67-immunoreactivity were also observed in all groups ( Fig. 5A , arrowheads in bottom panels).
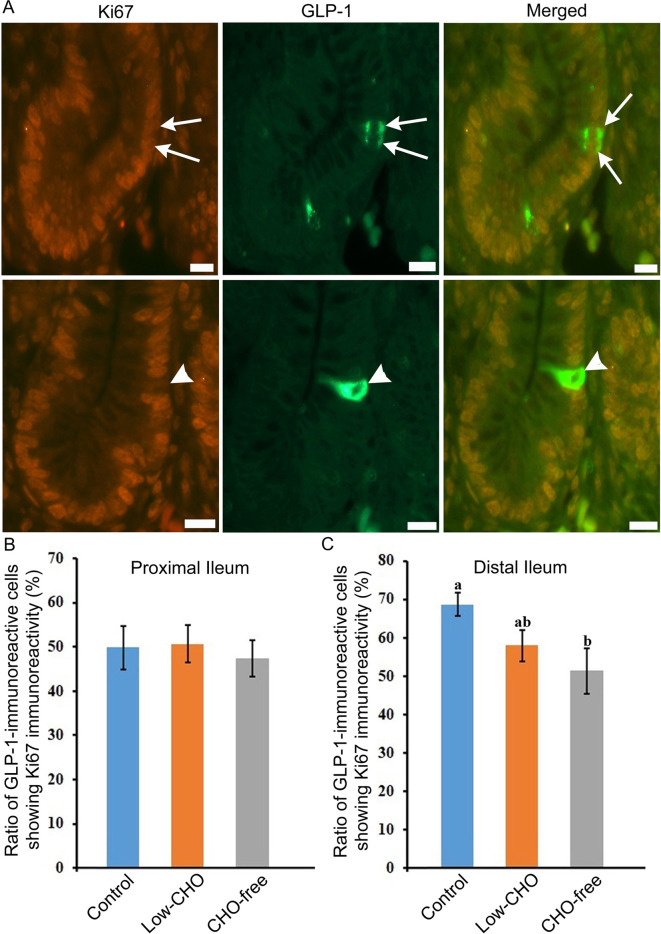
Glucagon-like peptide (GLP)-1-immunoreactive cells showing immunoreactivity for Ki-67 in the chicken distal ileum from the control group. A : Arrows in top panels indicate GLP-1-immunoreactive cells which also show immunoreactivity for Ki-67. Arrowheads in the bottom panels indicate immunoreactivity for GLP-1 but not for Ki-67. B : The ratio of GLP-1-immunoreactive cells showing Ki-67 in the proximal ileum from three groups. No significant difference in the ratio is observed among the three groups. C: The ratio of GLP-1-immunoreactive cells showing Ki-67 in the distal ileum from the control, low-carbohydrate (CHO), and CHO-free groups. There are significant differences between different alphabets. P <0.05. Error bars: Standard error. a>b.
The present study demonstrates that dietary CHO level influences the density of GLP-immunoreactive cells, or L cells, in the chicken ileum. Our previous study using transmission electron microscope images indicated that L cells in the chicken ileum were characterized by a long cytoplasmic process and microvilli on the apical surface. This study also revealed that GLP-1 and GLP-2 are costored in the same secretory granule [ 28 ]. These types of EECs, called open-type, act as chemosensors to detect intraluminal nutrients [ 12 ]. Furthermore, the microvilli of open-type EECs contain various receptors that respond to chemical signals from contents in the intestinal lumen [ 2 ]. Therefore, EECs, immunoreactive for GLP-1 and GLP-2, would function as chemical signal receptors for contents in the lumen.
Several reports have shown that ingested nutrients, such as CHOs, proteins, and fatty acids, have a variety of effects on L cells in the mammalian gut [ 5 , 20 , 40 ]. In addition, L cells release both GLP-1 and GLP-2 in response to glucose in the small intestine of rats [ 33 ]. Moreover, changes in dietary components impede GI hormone production, as do changes in EEC prevalence [ 11 ]. We have demonstrated that dietary protein and supplementation of amino acids influence the density of L cells in the chicken small intestine [ 24 , 29 ]. Moreover, our recent study indicated that dietary CHOs positively affected the proliferation of intestinal epithelial cells including enterocytes and goblet cells in the chicken ileum [ 34 ]. These findings suggest the close relationship of dietary nutrients with activities of epithelium in the chicken small intestine.
In the present study, the occurrence of GLP-1- and GLP-2-immunoreactive cells was significantly decreased in the two experimental groups, especially in the CHO-free group. Furthermore, GLP-1- and GLP-2-immunoreactive cells exhibited an oval or round shape were observed in the CHO-free group. Such cells were mostly seen in the fasting chicken ileum, where they form vacuoles with tiny lobule nuclei in the perikaryon (our unpublished data). An ultrastructural study demonstrated fasting-induced degeneration in epithelial cell components of the small intestine of White Leghorn chicken [ 39 ]. As a consequence of these findings, the phenomenon implies that a lack of luminal CHO leads to L cell degeneration. This might explain why the frequency of GLP-immunoreactive cells decreased in the lower CHO groups in the present study. Our previous studies [ 17 , 24 ] demonstrated that the effect of dietary protein on GLP-2-immunoreactivee cells was opposite to that on GLP-1-immunoreactive cells. However, there was no difference in the effect of dietary carbohydrate on these cells in this study. This is a reasonable result because GLP-1 and GLP-2 are co-stored in the same secretory granule of the chicken intestinal L cell [ 28 ].
Prohormone convertase (PC) enzymes mediate tissue-specific post-translational processing, which liberates peptide hormones in a tissue-specific way. PC1/3 enzymatic modifications in intestinal L cells generate GLP-1 and GLP-2 peptide hormones from PG [ 4 , 9 , 10 ]. However, studies conducted on GLUTag cell culture, or GLP-1 secreting cells, have revealed that glucose is the most efficient stimulator for regulating PG transcription [ 7 , 30 ]. Additionally, various nondigestible CHOs and their derivatives may upregulate the PG transcription level in the distal mammalian gut [ 5 , 41 ]. Taken together, CHO levels significantly impact the expression of PG mRNA in a variety of animal species. This study demonstrated that the prevalence of cells expressing PG mRNA signal decreased in the CHO-free group compared with that in the control group. Therefore, the present findings suggest that CHO may control PG transcription in the ileum.
Ki-67 is a nonhistone nuclear protein expressed in the G1, S, G2 and M phases of the cell cycle [ 22 ], generating its usefulness as a proliferation marker. This study evaluated the coexpression of Ki-67 and GLP-1 in cells from the proximal and distal ileum. The ratio of coexpressive cells was not impacted by dietary CHO level in the proximal ileum. However, the distal ileum showed a decreasing trend in the ratio of coexpressive cells with the reduction of dietary CHO level. A significant difference was measured between the control and CHO-free groups. These findings coincide with the results of PG mRNA expression mentioned above. Our previous findings indicated the upregulating effect of dietary CHO on the proliferation of epithelial cells in the chicken distal ileum [ 34 ]. Results of this study, therefore, suggest that dietary CHO also effectively activates L cell proliferation in the chicken distal ileum.
The number of L cells gradually increased from proximal to distal parts of the chicken small intestine, with the highest frequency found in the distal ileum [ 18 , 19 ]. GLP-2 is a trophic hormone released from L cell and has been shown to stimulate epithelial cell proliferation [ 8 ]. Reimann et al. [ 31 ] demonstrated that the facilitative glucose transporter2 (GLUT2) was involved in the regulation of the hormone release from rodent L cells. Though there has been no data showing the expression of GLUT2 in chicken intestinal L cells, it is hypothesized that the high number of L cells may secrete higher amount GLP-2 which in turn actively stimulates L cells proliferation in the distal ileum in the present study. This issue will need more systematic investigation in order to be addressed.
Corn oil and cellulose were included at a higher level in all groups’ diets to maintain isoenergic conditions. Obviously, increasing the concentration of these substances had no significant effect on any of the parameters investigated in this study; if they had, they would be seen in the CHO-free group.
In conclusion, dietary CHO could play a key role in stimulating activities and L cell proliferation in the chicken ileum. Indeed, the results presented here provide evidence that dietary CHO level influences the density of L cells in the chicken small intestine.
CONFLICTS OF INTEREST
There are no conflicts of interest to declare.
- 1. Balkan B.2000. Effects of glucagon-like peptide-1 (GLP-1) on glucose homeostasis and food intake. Appetite 35: 269–270. doi: 10.1006/appe.2000.0354 [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Breer H., Eberle J., Frick C., Haid D., Widmayer P.2012. Gastrointestinal chemosensation: chemosensory cells in the alimentary tract. Histochem. Cell Biol. 138: 13–24. doi: 10.1007/s00418-012-0954-z [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Brubaker P. L.2006. The glucagon-like peptides: pleiotropic regulators of nutrient homeostasis. Ann. N. Y. Acad. Sci. 1070: 10–26. doi: 10.1196/annals.1317.006 [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Burrin D. G., Stoll B., Guan X.2003. Glucagon-like peptide 2 function in domestic animals. Domest. Anim. Endocrinol. 24: 103–122. doi: 10.1016/S0739-7240(02)00210-2 [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Cani P. D., Hoste S., Guiot Y., Delzenne N. M.2007. Dietary non-digestible carbohydrates promote L-cell differentiation in the proximal colon of rats. Br. J. Nutr. 98: 32–37. doi: 10.1017/S0007114507691648 [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Cheeseman C. I., Tsang R.1996. The effect of GIP and glucagon-like peptides on intestinal basolateral membrane hexose transport. Am. J. Physiol. 271: G477–G482. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Daoudi M., Hennuyer N., Borland M. G., Touche V., Duhem C., Gross B., Caiazzo R., Kerr-Conte J., Pattou F., Peters J. M., Staels B., Lestavel S.2011. PPARβ/δ activation induces enteroendocrine L cell GLP-1 production. Gastroenterology 140: 1564–1574. doi: 10.1053/j.gastro.2011.01.045 [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Drucker D. J.2003. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol. Endocrinol. 17: 161–171. doi: 10.1210/me.2002-0306 [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Drucker D. J.2005. Biologic actions and therapeutic potential of the proglucagon-derived peptides. Nat. Clin. Pract. Endocrinol. Metab. 1: 22–31. doi: 10.1038/ncpendmet0017 [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Drucker D. J.2006. The biology of incretin hormones. Cell Metab. 3: 153–165. doi: 10.1016/j.cmet.2006.01.004 [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. El-Salhy M., Mazzawi T., Hausken T., Hatlebakk J. G.2016. Interaction between diet and gastrointestinal endocrine cells. Biomed. Rep. 4: 651–656. doi: 10.3892/br.2016.649 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Gribble F. M., Reimann F.2016. Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu. Rev. Physiol. 78: 277–299. doi: 10.1146/annurev-physiol-021115-105439 [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Guan X., Karpen H. E., Stephens J., Bukowski J. T., Niu S., Zhang G., Stoll B., Finegold M. J., Holst J. J., Hadsell D., Nichols B. L., Burrin D. G.2006. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology 130: 150–164. doi: 10.1053/j.gastro.2005.11.005 [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Guesdon J. L., Ternynck T., Avrameas S.1979. The use of avidin-biotin interaction in immunoenzymatic techniques. J. Histochem. Cytochem. 27: 1131–1139. doi: 10.1177/27.8.90074 [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Hiramatsu K.2020. Chicken intestinal L cells and glucagon-like peptide-1 secretion. J. Poult. Sci. 57: 1–6. doi: 10.2141/jpsa.0190003 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Hiramatsu K., Ohshima K.1995. Immunohistochemical study on the distribution of galanin-containing nerves in the chicken pancreas. Histol. Histopathol. 10: 283–288. [ PubMed ] [ Google Scholar ]
- 17. Hiramatsu K., Nishimura K., Kita K.2022. Influences of dietary amino acids supplementation on L cells in the chicken small intestine. World’s Poultry Congress 2022 in Paris . In press.
- 18. Hiramatsu K., Yamasaki A., Karasawa Y.2003. Comparative study on the distribution of glucagon-like peptide-1 (GLP-1)-immunoreactive cells in the intestine of chicken and ostrich. J. Poult. Sci. 40: 39–44. doi: 10.2141/jpsa.40.39 [ DOI ] [ Google Scholar ]
- 19. Hiramatsu K., Yamasaki A., Shioji T.2005. Immunohistochemical and morphometrical studies on the distribution of glucagon-like peptide-1 (GLP-1)-immunoreactive cells in the chicken intestine. J. Poult. Sci. 42: 223–229. doi: 10.2141/jpsa.42.223 [ DOI ] [ Google Scholar ]
- 20. Karhunen L. J., Juvonen K. R., Huotari A., Purhonen A. K., Herzig K. H.2008. Effect of protein, fat, carbohydrate and fibre on gastrointestinal peptide release in humans. Regul. Pept. 149: 70–78. doi: 10.1016/j.regpep.2007.10.008 [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Kissow H., Hartmann B., Holst J. J., Viby N. E., Hansen L. S., Rosenkilde M. M., Hare K. J., Poulsen S. S.2012. Glucagon-like peptide-1 (GLP-1) receptor agonism or DPP-4 inhibition does not accelerate neoplasia in carcinogen treated mice. Regul. Pept. 179: 91–100. doi: 10.1016/j.regpep.2012.08.016 [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Landberg G., Tan E. M., Roos G.1990. Flow cytometric multiparameter analysis of proliferating cell nuclear antigen/cyclin and Ki-67 antigen: a new view of the cell cycle. Exp. Cell Res. 187: 111–118. doi: 10.1016/0014-4827(90)90124-S [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Latorre R., Sternini C., De Giorgio R., Greenwood-Van Meerveld B.2016. Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol. Motil. 28: 620–630. doi: 10.1111/nmo.12754 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Monir M. M., Hiramatsu K., Matsumoto S., Nishimura K., Takemoto C., Shioji T., Watanabe T., Kita K., Yonekura S., Roh S. G.2014. Influences of protein ingestion on glucagon-like peptide (GLP)-1-immunoreactive endocrine cells in the chicken ileum. Anim. Sci. J. 85: 581–587. doi: 10.1111/asj.12177 [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Monir M. M., Hiramatsu K., Nishimura K., Takemoto C., Watanabe T.2014. Distribution of glucagon-like peptide (GLP)-2-immunoreactive cells in the chicken small intestine: antigen retrieval immunohistochemistry. J. Vet. Med. Sci. 76: 565–568. doi: 10.1292/jvms.13-0513 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. National Agriculture and Food Research Organization (NARO).2011. Japanese Feeding Standard for Poultry. Japan Livestock Industry Association, Tokyo. [ Google Scholar ]
- 27. Nauck M. A.1998. Glucagon-like peptide 1 (GLP-1): a potent gut hormone with a possible therapeutic perspective. Acta Diabetol. 35: 117–129. doi: 10.1007/s005920050116 [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Nishimura K., Hiramatsu K., Monir M. M., Takemoto C., Watanabe T.2013. Ultrastructural study on colocalization of glucagon-like peptide (GLP)-1 with GLP-2 in chicken intestinal L-cells. J. Vet. Med. Sci. 75: 1335–1339. doi: 10.1292/jvms.13-0106 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Nishimura K., Hiramatsu K., Watanabe T., Makino R., Sasaki N., Kita K.2015. Amino acid supplementation to diet influences the activity of the L cells in chicken small intestine. J. Poult. Sci. 52: 221–226. doi: 10.2141/jpsa.0150031 [ DOI ] [ Google Scholar ]
- 30. Puddu A., Sanguineti R., Montecucco F., Viviani G. L.2014. Glucagon-like peptide-1 secreting cell function as well as production of inflammatory reactive oxygen species is differently regulated by glycated serum and high levels of glucose. Mediators Inflamm. 2014: 923120. doi: 10.1155/2014/923120 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Reimann F., Habib A. M., Tolhurst G., Parker H. E., Rogers G. J., Gribble F. M.2008. Glucose sensing in L cells: a primary cell study. Cell Metab. 8: 532–539. doi: 10.1016/j.cmet.2008.11.002 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Richards M. P., McMurtry J. P.2008. Expression of proglucagon and proglucagon-derived peptide hormone receptor genes in the chicken. Gen. Comp. Endocrinol. 156: 323–338. doi: 10.1016/j.ygcen.2008.01.014 [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Roberge J. N., Brubaker P. L.1993. Regulation of intestinal proglucagon-derived peptide secretion by glucose-dependent insulinotropic peptide in a novel enteroendocrine loop. Endocrinology 133: 233–240. doi: 10.1210/endo.133.1.8319572 [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Salahuddin M., Hiramatsu K., Tamura K., Kita K.2021. Dietary carbohydrate effects on histological features of ileal mucosa in White Leghorn chicken. J. Vet. Med. Sci. 83: 952–956. doi: 10.1292/jvms.21-0157 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Thulesen J.2004. Glucagon-like peptide 2 (GLP-2), an intestinotrophic mediator. Curr. Protein Pept. Sci. 5: 51–65. doi: 10.2174/1389203043486946 [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Tolessa T., Gutniak M., Holst J. J., Efendic S., Hellström P. M.1998. Glucagon-like peptide-1 retards gastric emptying and small bowel transit in the rat: effect mediated through central or enteric nervous mechanisms. Dig. Dis. Sci. 43: 2284–2290. doi: 10.1023/A:1026678925120 [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Watanabe T., Nishimura K., Hosaka Y. Z., Shimosato T., Yonekura S., Suzuki D., Takemoto C., Monir M. M., Hiramatsu K.2014. Histological analysis of glucagon-like peptide-1 receptor expression in chicken pancreas. Cell Tissue Res. 357: 55–61. doi: 10.1007/s00441-014-1836-6 [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Wøjdemann M., Wettergren A., Hartmann B., Hilsted L., Holst J. J.1999. Inhibition of sham feeding-stimulated human gastric acid secretion by glucagon-like peptide-2. J. Clin. Endocrinol. Metab. 84: 2513–2517. doi: 10.1210/jcem.84.7.5840 [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Yamauchi K., Kamisoyama H., Isshiki Y.1996. Effects of fasting and refeeding on structures of the intestinal villi and epithelial cells in White Leghorn hens. Br. Poult. Sci. 37: 909–921. doi: 10.1080/00071669608417922 [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Yoder S. M., Yang Q., Kindel T. L., Tso P.2009. Stimulation of incretin secretion by dietary lipid: is it dose dependent? Am. J. Physiol. Gastrointest. Liver Physiol. 297: G299–G305. doi: 10.1152/ajpgi.90601.2008 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Zhou J., Martin R. J., Tulley R. T., Raggio A. M., McCutcheon K. L., Shen L., Danna S. C., Tripathy S., Hegsted M., Keenan M. J.2008. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am. J. Physiol. Endocrinol. Metab. 295: E1160–E1166. doi: 10.1152/ajpendo.90637.2008 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (16.3 MB)
- Collections

Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Download Free PDF
Chicken ileum: a better option for conducting isolated tissue experiments and bioassay

2017, IP Innovative Publication Pvt. Ltd.
In vivo method of animal testing is the use of non-human animals in experiments. Mice, rats, rabbits, guinea pigs, hamsters and non-human primates are widely used as laboratory animals. In vitro cell culture technique and in silico computer simulation are alternatives to in vivo animal testing. Current limitations on performing experiments with laboratory animals bring about the need for search of alternate tissues for biological testing. Bioassay is an integral part of undergraduate and postgraduate Pharmacology curriculum where various isolated tissues like frog rectus, rat colon, guinea pig ileum etc. were used for which whole animal has to be sacrificed for a small piece of tissue. So, we conducted bioassay of acetylcholine on chicken ileum obtained from chicken sacrificed for food which is easily available from slaughter houses. We found greater height of response and stability with Tyrode solution compared with Ringer Locke solution. We assessed the unknown concentration of acetylcholine by interpolation method of bioassay which we found 82.17% correct of actual concentration of unknown. Isolated chicken ileum is a better option available for conducting isolated tissue experiments considering the restriction with the use of experimental animals under CPCSEA guidelines.
Related papers
Journal of Advanced Pharmaceutical Technology & Research, 2012
Pharmacology as a subject depends largely on experiments conducted in laboratory animals. Experimental animals like rat, guinea pig, rabbit, etc. are used for the biological assay. For the teaching purposes to use isolated strip preparations from various organs, the laboratory animal species has to be sacrificed just for a piece of tissue. The present study was aimed to develop ex vivo model for pharmacological experimentation, which will mimic the actual laboratory condition without sacrificing the experimental animals. Dose response curve of acetylcholine alone and in presence of different concentrations of atropine was plotted using isolated chicken ileum, chicken duodenum, rat ileum, and rat duodenum and their EC 50 values were compared. The effect of atropine in terms of its type of antagonism was predicted based on Schild plot and pA 2 values were obtained. The chicken ileum and duodenum were also evaluated for four-and three-point bioassay, respectively. The results suggested that acetylcholine produced a dose-dependent increase in contraction in both chicken and rat ileum and duodenum preparation. The concentration response curve of acetylcholine in chicken ileum shifted toward left side of rat ileum with a higher EC 50 value. Atropine shifted the concentration response curve of acetylcholine toward right with a change in EC 50 value. Schild plots indicated that antagonism produced by atropine was found to be competitive in nature. The pA 2 values of atropine were found significantly high with isolated chicken ileum as compared to rat ileum preparation. It is concluded that isolated chicken ileum and duodenum preparation can be employed for routine experiments of pharmacology subject and the use of these isolated preparations is a novel approach for managing pharmacological experiments and importantly, without sacrificing the experimental animals.
Studium Press (India) Pvt. Ltd. ISBN 1: 978-93-85046-42-1, 2019
• This book provides fundamental knowledge of practical aspects of the experimental pharmacology right from laboratory animal handling and tissue mounting to practical implications of various important complex experimental procedures. • This book covers experiments from New PCI Syllabus for B. Pharmacy (education regulation 2014) in addition to that the important techniques and procedures used in experimental pharmacology. • This book is prepared using simple language and tried to explain the experiment with a model data of observation, calculation, graph and result for better clarity to carry out the experiments in animals or understand the experimental pharmacological techniques by observing the simulated experiments. • The experimental methodology and experimental data explained in this book are designed on the basis of robust scientific materials and personal experience in hands-on experiments under the guidance of eminent personalities. • This book will be helpful for graduates and postgraduates related to pharmacology, trainees, research workers during their day-to-day activities. Several simple and newer experimental models have been incorporated which may help the students to engage in drug discovery activities in the future. Besides this, several important points have been discussed e.g. ethics of animal experimentation, care, and handling of experimental animals, preparation of solutions, tissue mounting for in vitro studies, etc.
The Indian journal of medical research, 1984
When acetylcholine (ACh) 500 P,g/ml was perfused through lumen of isolated guineapig ileum for 5 min, it passed into the bath fluid and caused contraction ofa test piece of ileum. With everted pieces of guineapig ileum, the serosal surface forming the lumen was perfused with Tyrode's solution, while exposing the mucosal surface facing the bath fluid to 400, 800 u 1600 p.g/ml ACh for 10 min and the perfusate was biologically assayed for ACh on frog rectus muscle. ACh passed from the mucosal to the serosal surface with a gradient of 300 to 800 : 1 which was reduced to 200 to 400 : 1 after. eserinization. ACh, Smg or 10 mg/ml instilled into the eye of anaesthetized dog for 10 min contracted the pupil and was detected chemically in the aqueous humour at a gradient of 200 : 1 .
Alternatives to Laboratory Animals, 2010
A study was undertaken to determine the longevity of active muscarinic receptors on abattoir-sourced isolated ileum preparations from Gallus gallus domesticus, with a view to using the tissue as an experimental tool for functional response assays in laboratory experiments. A concentration-response curve for acetylcholine (1-256μM) was plotted, in the presence and absence of 1, 3 and 6nM atropine. In a second experiment, unknown concentrations of acetylcholine samples were determined by using an interpolation method. In this experiment, four sample concentrations were used and the calculated values were found to be almost equal to the actual values. Finally, an experiment was carried out to elucidate the effects of post-sacrifice time on the contractile response of the tissue. The results showed that the tissue maintained considerable contractile response at the 6-hour post-sacrifice time-point. Competitive antagonistic activity was observed between acetylcholine and atropine on the chicken ileum, and the pA2 value was calculated to be 9.21 by using an Arunlakshana-Schild plot. The results suggest that isolated ileum preparations of Gallus gallus domesticus, obtained from a meat abattoir, can be used as a basic experimental tool for bioassays in routine laboratory experiments. However, its potential as a research tool still needs to be confirmed.
Phytochemical Analysis, 2003
A method has been developed to determine the false-positive effects on acetylcholinesterase inhibition in the TLC assay based on Ellman's method. Various aldehydes and amines have been tested in order to determine whether the observed inhibition is due to a true enzyme inhibition or due to the inhibition of the reaction between thiocholine and 5,5′-dithiobis-(2-nitrobenzoic acid). 4-Dimethylaminobenzaldehyde, 3-ethoxy-4-hydroxybenzaldehyde, diethylamine, triethylamine, triethanolamine and tyramine showed real enzyme inhibition, although their activity was about 103 times lower than that shown by galanthamine. Heptanal, decanal, cinnamaldehyde, anisaldehyde, benzaldehyde, hexylamine and tryptamine appeared to show a non-specific chemical inhibition. By checking this chemical inhibition on the TLC assay, the true enzyme inhibition could be distinguished from the false-positive chemical inhibition observed in the toluene extract of Nerine bowdenii in the course of isolation of active compounds. Copyright © 2003 John Wiley & Sons, Ltd.
Journal of Drug Delivery and Therapeutics
In this era where the pharmaceutical companies and products are hiking in its need and production it is inevitable to document the safety and toxicity along with the indications of the same. This is where the experimental study has a vital role to play. Experimental pharmacology is the science where the drug interaction with different receptors and target sites in living organism are explained. This article reviews about the different aspects of experimental pharmacology and its uses. Keywords: Experimental pharmacology, products, dosage
The purpose of this study was to characterized the pharmacokinetic of AMX after intravenous (IV), intramuscular (IM) and subcutaneous (SC) administration in adult llamas. Six female llamas (110,17 ± 25,17 Kg) recieved AMX (sodium salt, 20 mg/kg) by each route with two weeks washout period. Serial venous blood samples were taken at predeterminated times after drug administration. AMX plasma concentration were determined by microbiological assay, using Bacillus subtilis ATCC 6633 as test microorganism. Plasma disposition curves were analized using Topfit software. After IM and SC route AMX was totally avaible with an F value around 150% and 112%, respectively. C max for IM administration was 40.38 ± 12.08 μg/ml. and T max 0.30 ± 0.22 h. After SC administration Cmax was significant lower 12.51± 5.37 μg/ml and Tmax later 0.78 ± 0.42 h, but serum concentration stayed longer (MRTsc 3.21 ± 1.71 h) than for IM route (MRTim 1.37 ± 0.51 h). Total body clearence and volumen of distribution for the IV route were 9.07 ± 2.12 ml/h and 0.73 ± 0.19, respectively. Terminal halflife was 0.94 ± 0.13 h, 0.86 ± 0.28 h, 2.23 ± 1.18 h for the IV, IM, SC routes, respectively. Mean residence time for the IV route was 1.07 ± 0.30 h. These results show differences in some AMX pharmacokinetic parameters when administred by the IV, IM and SC routes. O2. PHARMACOKINETIC OF INTRAVENOUS CEPHAL-OTHIN IN CATS
Background: Endometriosis refers the presence of endometrial tissues both gland and stroma other than the uterine cavity. Endometriosis often presents with nonspecific symptoms like chronic pelvic pain, dysmenorrhea, and infertility, making clinical diagnosis challenging. Laparoscopy, the gold standard for diagnosing endometriosis, allows direct visualization of endometrial implants. Understanding its prevalence can enhance diagnostic and therapeutic approaches. This study aimed to assess the prevalence of endometriosis in women undergoing laparoscopic surgery for various gynecological indications. Methods: This retrospective study was conducted in the Department of Gynae & Obs, Uttara Adhunik Medical College Hospital, Dhaka, Bangladesh from January 2021 to December 2021. A total of 117 cases undergoing various laparoscopic procedures were enrolled in this study. A random sampling technique was used for sample selection, and data were analyzed using MS Office tools.
FUDMA JOURNAL OF MANAGEMENT SCIENCES, 2021
Gephyra 18, 2019
Italia settentrionale e regioni dell'arco alpino tra V e VI secolo d.C. Atti del convegno (15-17 aprile 2021), 2022
Meriç Uluslararası Sosyal ve Stratejik Araştırmalar Dergisi- Cilt: 3 - Sayı: 7, 2019
Castalia - Revista De Psicología De La Academia, 2024
En: Muñoz, I. y Osandón, L. [Comp.], La didáctica de la Historia y la Formación de Ciudadanos en el mundo actual, 2013
Sociologie dětství a dítěte. Distanční studijní text, 2019
Klinička psihologija, 2016
Journal La Edusci, 2024
RECIE Revista electrónica científica de investigación educativa, 2023
Atem / Breath, 2021
African Safety Promotion, 2016
DOAJ (DOAJ: Directory of Open Access Journals), 2014
UMRAN - International Journal of Islamic and Civilizational Studies
Journal of International Education Research (JIER), 2013
akademik.unsri.ac.id
Pharmacological Research, 1992
Revista de Biología Tropical, 2021
Dental Materials, 1995
Obesity Facts, 2010
Related topics
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
Indian Journal of Pharmacy and Pharmacology
Official Publication of Innovative Education and Scientific Research Foundation
Published by IP Innovative Publication Pvt. Ltd.

Print ISSN: 2393-9079
Online ISSN: 2393-9087
CODEN : IJPPTK
- Current Issue
Volume: 11 , Issue: 3
Article type
Review Article
Article page
Authors details.
VM Motghare , MB Nandeshwar , CS Bajait , SA Pimpalkhute , SD Sontakke
View Article As
Downlaod files.
Bookmark article
Share article
Article indexing.
Citation Managers
Download Citation
Article statistics
Viewed: 3698
PDF Downloaded: 3934
Chicken ileum: a better option for conducting isolated tissue experiments and bioassay
Abstract Full Text PDF -----> Share on Facebook Share on Twitter -->